Phantomless calibration of CT scans for hip fracture risk prediction in silico: Comparison with phantom-based calibration
- PMID: 38875268
- PMCID: PMC11178222
- DOI: 10.1371/journal.pone.0305474
Phantomless calibration of CT scans for hip fracture risk prediction in silico: Comparison with phantom-based calibration
Abstract
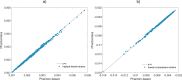
Finite element models built from quantitative computed tomography images rely on element-wise mapping of material properties starting from Hounsfield Units (HU), which can be converted into mineral densities upon calibration. While calibration is preferably carried out by scanning a phantom with known-density components, conducting phantom-based calibration may not always be possible. In such cases, a phantomless procedure, where the scanned subject's tissues are used as a phantom, is an interesting alternative. The aim of this study was to compare a phantom-based and a phantomless calibration method on 41 postmenopausal women. The proposed phantomless calibration utilized air, adipose, and muscle tissues, with reference equivalent mineral density values of -797, -95, and 38 mg/cm3, extracted from a previously performed phantom-based calibration. A 9-slice volume of interest (VOI) centred between the femoral head and knee rotation centres was chosen. Reference HU values for air, adipose, and muscle tissues were extracted by identifying HU distribution peaks within the VOI, and patient-specific calibration was performed using linear regression. Comparison of FE models calibrated with the two methods showed average relative differences of 1.99% for Young's modulus1.30% for tensile and 1.34% for compressive principal strains. Excellent correlations (R2 > 0.99) were identified for superficial maximum tensile and minimum compressive strains. Maximum normalised root mean square relative error (RMSRE) values settled at 4.02% for Young's modulus, 2.99% for tensile, and 3.22% for compressive principal strains, respectively. The good agreement found between the two methods supports the adoption of the proposed methodology when phantomless calibration is needed.
Copyright: © 2024 Szyszko et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Calibration with or without phantom for fracture risk prediction in cancer patients with femoral bone metastases using CT-based finite element models.PLoS One. 2019 Jul 30;14(7):e0220564. doi: 10.1371/journal.pone.0220564. eCollection 2019. PLoS One. 2019. PMID: 31361790 Free PMC article.
-
Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision.Bone. 2017 Oct;103:325-333. doi: 10.1016/j.bone.2017.07.029. Epub 2017 Aug 1. Bone. 2017. PMID: 28778598 Free PMC article.
-
The effect of variations in CT scan protocol on femoral finite element failure load assessment using phantomless calibration.PLoS One. 2022 Mar 18;17(3):e0265524. doi: 10.1371/journal.pone.0265524. eCollection 2022. PLoS One. 2022. PMID: 35303026 Free PMC article.
-
Lumbar Bone Mineral Density Phantomless Computed Tomography Measurements and Correlation with Age and Fracture Incidence.Traffic Inj Prev. 2015;16 Suppl 2(0 2):S153-60. doi: 10.1080/15389588.2015.1054029. Traffic Inj Prev. 2015. PMID: 26436225 Free PMC article.
-
Application of quantitative computed tomography for assessment of trabecular bone mineral density, microarchitecture and mechanical property.Clin Imaging. 2016 Mar-Apr;40(2):330-8. doi: 10.1016/j.clinimag.2015.09.016. Epub 2015 Sep 21. Clin Imaging. 2016. PMID: 26602163 Review.
Cited by
-
Predictive efficacy of phantom-less calibration of computed tomography for cage subsidence after anterior cervical discectomy and fusion.Eur Spine J. 2025 Aug 23. doi: 10.1007/s00586-025-09284-z. Online ahead of print. Eur Spine J. 2025. PMID: 40848160
References
-
- Jeremiah MP, Unwin BK, Greenawald MH, Casiano VE. Diagnosis and Management of Osteoporosis. Am Fam Physician. 2015. Aug;92(4):261–8. - PubMed
-
- Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. Erratum in: Osteoporos Int. 2020 Jan;31(1):209. Erratum in: Osteoporos Int. 2020 Apr;31(4):801. doi: 10.1007/s00198-018-4704-5 - DOI - PMC - PubMed

