Constructing static two-electron lithium-bromide battery
- PMID: 38875345
- PMCID: PMC11177945
- DOI: 10.1126/sciadv.adl0587
Constructing static two-electron lithium-bromide battery
Abstract
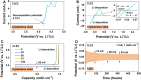
Despite their potential as conversion-type energy storage technologies, the performance of static lithium-bromide (SLB) batteries has remained stagnant for decades. Progress has been hindered by the intrinsic liquid-liquid redox mode and single-electron transfer of these batteries. Here, we developed a high-performance SLB battery based on the active bromine salt cathode and the two-electron transfer chemistry with a Br-/Br+ redox couple by electrolyte tailoring. The introduction of NO3- improved the reversible single-electron transition of Br-, and more impressively, the coordinated Cl- anions activated the Br+ conversion to provide an additional electron transfer. A voltage plateau was observed at 3.8 V, and the discharge capacity and energy density were increased by 142 and 159% compared to the one-electron reaction benchmark. This two-step conversion mechanism exhibited excellent stability, with the battery functioning for 1000 cycles. These performances already approach the state of the art of currently established Li-halogen batteries. We consider the established two-electron redox mechanism highly exemplary for diversified halogen batteries.
Figures





Similar articles
-
Two-Electron Redox Chemistry Enabled High-Performance Iodide-Ion Conversion Battery.Angew Chem Int Ed Engl. 2022 Feb 21;61(9):e202113576. doi: 10.1002/anie.202113576. Epub 2022 Jan 12. Angew Chem Int Ed Engl. 2022. PMID: 34931752
-
High Capacity and Ultralong Lifespan Aqueous Lithium-Bromine Batteries Realized by Low-Cost Concentrated Electrolyte Coupled with Dependable Lithium Titanium Phosphate.ACS Appl Mater Interfaces. 2025 Feb 5;17(5):7773-7783. doi: 10.1021/acsami.4c19227. Epub 2025 Jan 24. ACS Appl Mater Interfaces. 2025. PMID: 39851216
-
Aqueous Alkaline Zinc-Iodine Battery with Two-Electron Transfer.ACS Nano. 2025 Jan 21;19(2):2900-2908. doi: 10.1021/acsnano.4c16550. Epub 2025 Jan 7. ACS Nano. 2025. PMID: 39772478
-
Lithium-Sulfur Batteries under Lean Electrolyte Conditions: Challenges and Opportunities.Angew Chem Int Ed Engl. 2020 Jul 27;59(31):12636-12652. doi: 10.1002/anie.201909339. Epub 2020 Mar 17. Angew Chem Int Ed Engl. 2020. PMID: 31490599 Review.
-
Advances in Lithium-Sulfur Batteries: From Academic Research to Commercial Viability.Adv Mater. 2021 Jul;33(29):e2003666. doi: 10.1002/adma.202003666. Epub 2021 Jun 6. Adv Mater. 2021. PMID: 34096100 Review.
Cited by
-
Immobilizing Zwitterionic Molecular Brush in Functional Organic Interfacial Layers for Ultra-Stable Zn-Ion Batteries.Nanomicro Lett. 2025 May 20;17(1):262. doi: 10.1007/s40820-025-01782-5. Nanomicro Lett. 2025. PMID: 40392345 Free PMC article.
-
Gram-Scale Synthesis and Optical Properties of Self-Trapped-Exciton-Emitting Two-Dimensional Tin Halide Perovskites.Nanomaterials (Basel). 2025 May 28;15(11):818. doi: 10.3390/nano15110818. Nanomaterials (Basel). 2025. PMID: 40497866 Free PMC article.
References
-
- Lu Y., Chen J., Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 4, 127–142 (2020). - PubMed
-
- Pomerantseva E., Bonaccorso F., Feng X., Cui Y., Gogotsi Y., Energy storage: The future enabled by nanomaterials. Science 366, eaan8285 (2019). - PubMed
-
- Ye Y. S., Chou L. Y., Liu Y. Y., Wang H. S., Lee H. K., Huang W. X., Wan J. Y., Liu K., Zhou G. M., Yang Y. F., Yang A. K., Xiao X., Gao X., Boyle D. T., Chen H., Zhang W. B., Kim S. C., Cui Y., Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 5, 786–793 (2020).
-
- Liu B., Zhang J. G., Xu W., Advancing lithium metal batteries. Joule 2, 833–845 (2018).
LinkOut - more resources
Full Text Sources

