Transforming Rapid Diagnostic Tests for Precision Public Health: Open Guidelines for Manufacturers and Users
- PMID: 38875688
- PMCID: PMC11041428
- DOI: 10.2196/26800
Transforming Rapid Diagnostic Tests for Precision Public Health: Open Guidelines for Manufacturers and Users
Abstract
Background: Precision public health (PPH) can maximize impact by targeting surveillance and interventions by temporal, spatial, and epidemiological characteristics. Although rapid diagnostic tests (RDTs) have enabled ubiquitous point-of-care testing in low-resource settings, their impact has been less than anticipated, owing in part to lack of features to streamline data capture and analysis.
Objective: We aimed to transform the RDT into a tool for PPH by defining information and data axioms and an information utilization index (IUI); identifying design features to maximize the IUI; and producing open guidelines (OGs) for modular RDT features that enable links with digital health tools to create an RDT-OG system.
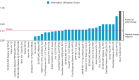
Methods: We reviewed published papers and conducted a survey with experts or users of RDTs in the sectors of technology, manufacturing, and deployment to define features and axioms for information utilization. We developed an IUI, ranging from 0% to 100%, and calculated this index for 33 World Health Organization-prequalified RDTs. RDT-OG specifications were developed to maximize the IUI; the feasibility and specifications were assessed through developing malaria and COVID-19 RDTs based on OGs for use in Kenya and Indonesia.
Results: The survey respondents (n=33) included 16 researchers, 7 technologists, 3 manufacturers, 2 doctors or nurses, and 5 other users. They were most concerned about the proper use of RDTs (30/33, 91%), their interpretation (28/33, 85%), and reliability (26/33, 79%), and were confident that smartphone-based RDT readers could address some reliability concerns (28/33, 85%), and that readers were more important for complex or multiplex RDTs (33/33, 100%). The IUI of prequalified RDTs ranged from 13% to 75% (median 33%). In contrast, the IUI for an RDT-OG prototype was 91%. The RDT open guideline system that was developed was shown to be feasible by (1) creating a reference RDT-OG prototype; (2) implementing its features and capabilities on a smartphone RDT reader, cloud information system, and Fast Healthcare Interoperability Resources; and (3) analyzing the potential public health impact of RDT-OG integration with laboratory, surveillance, and vital statistics systems.
Conclusions: Policy makers and manufacturers can define, adopt, and synergize with RDT-OGs and digital health initiatives. The RDT-OG approach could enable real-time diagnostic and epidemiological monitoring with adaptive interventions to facilitate control or elimination of current and emerging diseases through PPH.
Keywords: FHIR; Fast Healthcare Interoperability Resources; diagnostic; digital health; guideline; manufacture; precision public health; rapid diagnostic test; surveillance; testing.
©Peter Lubell-Doughtie, Shiven Bhatt, Roger Wong, Anuraj H Shankar. Originally published in JMIR Biomedical Engineering (http://biomsedeng.jmir.org), 29.07.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures





Similar articles
-
The use of Fionet technology for external quality control of malaria rapid diagnostic tests and monitoring health workers' performance in rural military health facilities in Tanzania.PLoS One. 2018 Dec 27;13(12):e0208583. doi: 10.1371/journal.pone.0208583. eCollection 2018. PLoS One. 2018. PMID: 30589853 Free PMC article.
-
Comparison of visual and automated Deki Reader interpretation of malaria rapid diagnostic tests in rural Tanzanian military health facilities.Malar J. 2018 May 29;17(1):214. doi: 10.1186/s12936-018-2363-9. Malar J. 2018. PMID: 29843721 Free PMC article.
-
Evaluation of malaria rapid diagnostic test (RDT) use by community health workers: a longitudinal study in western Kenya.Malar J. 2018 May 18;17(1):206. doi: 10.1186/s12936-018-2358-6. Malar J. 2018. PMID: 29776359 Free PMC article.
-
Force Protection Risks in AFRICOM, INDOPACOM and SOUTHCOM Due to Rapid Diagnostic Test Failures for Falciparum Malaria, 2016-2022.MSMR. 2023 Oct 1;30(10):7-11. MSMR. 2023. PMID: 37963222 Review.
-
Implementing COVID-19 (SARS-CoV-2) Rapid Diagnostic Tests in Sub-Saharan Africa: A Review.Front Med (Lausanne). 2020 Oct 30;7:557797. doi: 10.3389/fmed.2020.557797. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33195307 Free PMC article. Review.
References
-
- The Academy of Medical Sciences; 2016. Improving the Development and Deployment of Rapid Diagnostic Tests in LMICs. [2020-12-31]. https://acmedsci.ac.uk/file-download/21094033 .
-
- Chhetri A, Iversen M, Kaasbøll J, Kanjo C. Evaluating mHealth Apps Using Affordances: Case of CommCare Versus DHIS2 Tracker. In: Nielsen P, Kimaro HC. eds. Information and Communication Technologies for Development. Strengthening Southern-Driven Cooperation as a Catalyst for ICT4D. Vol 551. IFIP Advances in Information and Communication Technology. Springer International Publishing; 2019. pp. 619–632.
LinkOut - more resources
Full Text Sources
Miscellaneous

