Aurkin-A, a TPX2-Aurora A small molecule inhibitor disrupts Alisertib-induced polyploidy in aggressive diffuse large B cell lymphoma
- PMID: 38875929
- PMCID: PMC11225860
- DOI: 10.1016/j.neo.2024.101014
Aurkin-A, a TPX2-Aurora A small molecule inhibitor disrupts Alisertib-induced polyploidy in aggressive diffuse large B cell lymphoma
Abstract
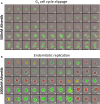
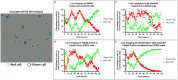
Chemotherapy induced polyploidy is a mechanism of inherited drug resistance resulting in an aggressive disease course in cancer patients. Alisertib, an Aurora Kinase A (AK-A) ATP site inhibitor, induces cell cycle disruption resulting in polyaneuploidy in Diffuse Large B Cell Lymphoma (DLBCL). Propidium iodide flow cytometry was utilized to quantify alisertib induced polyploidy in U2932 and VAL cell lines. In U2932 cells, 1µM alisertib generated 8n+ polyploidy in 48% of the total cell population after 5 days of treatment. Combination of Aurkin A an AK-A/TPX2 site inhibitor, plus alisertib disrupted alisertib induced polyploidy in a dose-dependent manner with associated increased apoptosis. We generated a stable FUCCI U2932 cell line expressing Geminin-clover (S/G2/M) and cdt1-mKO (G1), to monitor cell cycle progression. Using this system, we identified alisertib induces polyploidy through endomitosis, which was eliminated with Aurkin A treatment. In a VAL mouse xenograft model, we show polyploidy generation in alisertib treated mice versus vehicle control or Aurkin A. Aurkin A plus alisertib significantly reduced polyploidy to vehicle control levels. Our in vitro and in vivo studies show that Aurkin A synergizes with alisertib and significantly decreases the alisertib dose needed to disrupt polyploidy while increasing apoptosis in DLBCL cells.
Keywords: Alisertib; Aneuploidy; Cell cycle; Chromosomal instability; Polyploidy.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
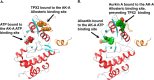





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

