Self-assembling 3D vessel-on-chip model with hiPSC-derived astrocytes
- PMID: 38876110
- PMCID: PMC11252484
- DOI: 10.1016/j.stemcr.2024.05.006
Self-assembling 3D vessel-on-chip model with hiPSC-derived astrocytes
Abstract
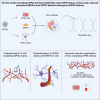
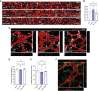
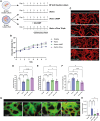
Functionality of the blood-brain barrier (BBB) relies on the interaction between endothelial cells (ECs), pericytes, and astrocytes to regulate molecule transport within the central nervous system. Most experimental models for the BBB rely on freshly isolated primary brain cells. Here, we explored human induced pluripotent stem cells (hiPSCs) as a cellular source for astrocytes in a 3D vessel-on-chip (VoC) model. Self-organized microvascular networks were formed by combining hiPSC-derived ECs, human brain vascular pericytes, and hiPSC-derived astrocytes within a fibrin hydrogel. The hiPSC-ECs and pericytes showed close interactions, but, somewhat unexpectedly, addition of astrocytes disrupted microvascular network formation. However, continuous fluid perfusion or activation of cyclic AMP (cAMP) signaling rescued the vascular organization and decreased vascular permeability. Nevertheless, astrocytes did not affect the expression of proteins related to junction formation, transport, or extracellular matrix, indicating that, despite other claims, hiPSC-derived ECs do not entirely acquire a BBB-like identity in the 3D VoC model.
Keywords: BBB; blood-brain barrier; hiPSC-Astro; hiPSC-ECs; hiPSC-derived astrocytes; hiPSC-derived endothelial cells; human induced pluripotent stem cells; microfluidics; organ-on-chip; vessel-on-chip.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Boyer-Di Ponio J., El-Ayoubi F., Glacial F., Ganeshamoorthy K., Driancourt C., Godet M., Perrière N., Guillevic O., Olivier Couraud P., Uzan G. Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084179. - DOI - PMC - PubMed
-
- Gastfriend B.D., Nishihara H., Canfield S.G., Foreman K.L., Engelhardt B., Palecek S.P., Shusta E.V. Wnt signaling mediates acquisition of blood–brain barrier properties in naïve endothelium derived from human pluripotent stem cells. Elife. 2021;10:1–33. doi: 10.7554/eLife.70992. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

