Pyroptosis: A spoiler of peaceful coexistence between cells in degenerative bone and joint diseases
- PMID: 38876191
- PMCID: PMC12126718
- DOI: 10.1016/j.jare.2024.06.010
Pyroptosis: A spoiler of peaceful coexistence between cells in degenerative bone and joint diseases
Abstract
Background: As people age, degenerative bone and joint diseases (DBJDs) become more prevalent. When middle-aged and elderly people are diagnosed with one or more disorders such as osteoporosis (OP), osteoarthritis (OA), and intervertebral disc degeneration (IVDD), it often signals the onset of prolonged pain and reduced functionality. Chronic inflammation has been identified as the underlying cause of various degenerative diseases, including DBJDs. Recently, excessive activation of pyroptosis, a form of programed cell death (PCD) mediated by inflammasomes, has emerged as a primary driver of harmful chronic inflammation. Consequently, pyroptosis has become a potential target for preventing and treating DBJDs.
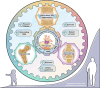
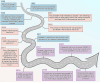
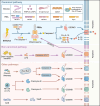
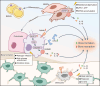
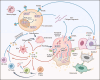
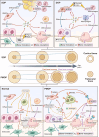
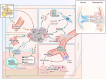
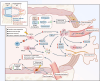
Aim of review: This review explored the physiological and pathological roles of the pyroptosis pathway in bone and joint development and its relation to DBJDs. Meanwhile, it elaborated the molecular mechanisms of pyroptosis within individual cell types in the bone marrow and joints, as well as the interplay among different cell types in the context of DBJDs. Furthermore, this review presented the latest compelling evidence supporting the idea of regulating the pyroptosis pathway for DBJDs treatment, and discussed the potential, limitations, and challenges of various therapeutic strategies involving pyroptosis regulation.
Key scientific concepts of review: In summary, an interesting identity for the unregulated pyroptosis pathway in the context of DBJDs was proposed in this review, which was undertaken as a spoiler of peaceful coexistence between cells in a degenerative environment. Over the extended course of DBJDs, pyroptosis pathway perpetuated its activity through crosstalk among pyroptosis cascades in different cell types, thus exacerbating the inflammatory environment throughout the entire bone marrow and joint degeneration environment. Correspondingly, pyroptosis regulation therapy emerged as a promising option for clinical treatment of DBJDs.
Keywords: Bone and joint diseases; Intervertebral disc degeneration; Osteoarthritis; Osteoporosis; Pyroptosis.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Natural Products in the Prevention of Degenerative Bone and Joint Diseases: Mechanisms Based on the Regulation of Ferroptosis.Phytother Res. 2025 Jan;39(1):162-188. doi: 10.1002/ptr.8366. Epub 2024 Nov 8. Phytother Res. 2025. PMID: 39513459 Review.
-
Pyroptosis in Skeleton Diseases: A Potential Therapeutic Target Based on Inflammatory Cell Death.Int J Mol Sci. 2024 Aug 21;25(16):9068. doi: 10.3390/ijms25169068. Int J Mol Sci. 2024. PMID: 39201755 Free PMC article. Review.
-
Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway.Biochem Biophys Res Commun. 2020 Jun 4;526(3):772-779. doi: 10.1016/j.bbrc.2020.03.161. Epub 2020 Apr 4. Biochem Biophys Res Commun. 2020. PMID: 32265028
-
ASIC1a mediated nucleus pulposus cells pyroptosis and glycolytic crosstalk as a molecular basis for intervertebral disc degeneration.Inflamm Res. 2025 Jan 28;74(1):29. doi: 10.1007/s00011-025-02003-w. Inflamm Res. 2025. PMID: 39870819 Review.
-
Pyroptosis and degenerative diseases of the elderly.Cell Death Dis. 2023 Feb 9;14(2):94. doi: 10.1038/s41419-023-05634-1. Cell Death Dis. 2023. PMID: 36755014 Free PMC article. Review.
Cited by
-
Pyroptosis: candidate key targets for mesenchymal stem cell-derived exosomes for the treatment of bone-related diseases.Stem Cell Res Ther. 2025 Feb 12;16(1):68. doi: 10.1186/s13287-025-04167-y. Stem Cell Res Ther. 2025. PMID: 39940049 Free PMC article. Review.
-
Epigenetic roles of chromatin remodeling complexes in bone biology and the pathogenesis of bone‑related disease (Review).Int J Mol Med. 2025 Aug;56(2):115. doi: 10.3892/ijmm.2025.5556. Epub 2025 May 30. Int J Mol Med. 2025. PMID: 40444490 Free PMC article. Review.
-
The Organ-Joint Axes in Osteoarthritis: Significant Pathogenesis and Therapeutic Targets.Aging Dis. 2024 Nov 21;16(5):2999-3021. doi: 10.14336/AD.2024.1223. Aging Dis. 2024. PMID: 39656496 Free PMC article. Review.
-
Metal ion-crosslinking multifunctional hydrogel microspheres with inflammatory immune regulation for cartilage regeneration.Front Bioeng Biotechnol. 2025 Jan 28;13:1540592. doi: 10.3389/fbioe.2025.1540592. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 39935604 Free PMC article.
-
The communication role of extracellular vesicles in the osteoarthritis microenvironment.Front Immunol. 2025 Mar 17;16:1549833. doi: 10.3389/fimmu.2025.1549833. eCollection 2025. Front Immunol. 2025. PMID: 40165965 Free PMC article. Review.
References
-
- Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. - PubMed
-
- Sims N.A., Gooi J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19(5):444–451. - PubMed
-
- Song S., Guo Y., Yang Y., Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. 2022;237 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

