Targeting colorectal cancer at the level of nuclear pore complex
- PMID: 38876192
- PMCID: PMC11976419
- DOI: 10.1016/j.jare.2024.06.009
Targeting colorectal cancer at the level of nuclear pore complex
Abstract
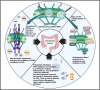
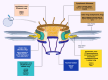
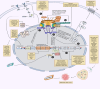
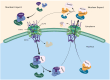
Background: Nuclear pore complexes (NPCs) are the architectures entrenched in nuclear envelop of a cell that regulate the nucleo-cytoplasmic transportation of materials, such as proteins and RNAs for proper functioning of a cell. The appropriate localization of proteins and RNAs within the cell is essential for its normal functionality. For such a complex transportation of materials across the NPC, around 60 proteins are involved comprising nucleoporins, karyopherins and RAN system proteins that play a vital role in NPC's structure formation, cargo translocation across NPC, and cargoes' rapid directed transportation respectively. In various cancers, the structure and function of NPC is often exaggerated, following altered expressions of its nucleoporins and karyopherins, affecting other proteins of associated signaling pathways. Some inhibitors of karyopherins at present, have potential to regulate the altered level/expression of these karyopherin molecules.
Aim of review: This review summarizes the data from 1990 to 2023, mainly focusing on recent studies that illustrate the structure and function of NPC, the relationship and mechanisms of nucleoporins and karyopherins with colorectal cancer, as well as therapeutic values, in order to understand the pathology and underlying basis of colorectal cancer associated with NPC. This is the first review to our knowledge elucidating the detailed updated studies targeting colorectal cancer at NPC. The review also aims to target certain karyopherins, Nups and their possible inhibitors and activators molecules as a therapeutic strategy.
Key scientific concepts of review: NPC structure provides understanding, how nucleoporins and karyopherins as key molecules are responsible for appropriate nucleocytoplasmic transportation. Many studies provide evidences, describing the role of disrupted nucleoporins and karyopherins not only in CRC but also in other non-hematological and hematological malignancies. At present, some inhibitors of karyopherins have therapeutic potential for CRC, however development of more potent inhibitors may provide more effective therapeutic strategies for CRC in near future.
Keywords: Colorectal cancer (CRC); Karyopherins; Nuclear pore complex (NPC); Nuclear transporters; Nucleocytoplasmic transport; Nucleoporins.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

