The bioenergetic landscape of cancer
- PMID: 38876266
- PMCID: PMC11259816
- DOI: 10.1016/j.molmet.2024.101966
The bioenergetic landscape of cancer
Abstract
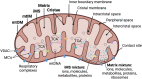
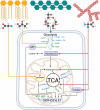
Background: Bioenergetic remodeling of core energy metabolism is essential to the initiation, survival, and progression of cancer cells through exergonic supply of adenosine triphosphate (ATP) and metabolic intermediates, as well as control of redox homeostasis. Mitochondria are evolutionarily conserved organelles that mediate cell survival by conferring energetic plasticity and adaptive potential. Mitochondrial ATP synthesis is coupled to the oxidation of a variety of substrates generated through diverse metabolic pathways. As such, inhibition of the mitochondrial bioenergetic system by restricting metabolite availability, direct inhibition of the respiratory Complexes, altering organelle structure, or coupling efficiency may restrict carcinogenic potential and cancer progression.
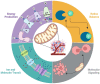
Scope of review: Here, we review the role of bioenergetics as the principal conductor of energetic functions and carcinogenesis while highlighting the therapeutic potential of targeting mitochondrial functions.
Major conclusions: Mitochondrial bioenergetics significantly contribute to cancer initiation and survival. As a result, therapies designed to limit oxidative efficiency may reduce tumor burden and enhance the efficacy of currently available antineoplastic agents.
Keywords: Bioenergetics; Cancer; Cell survival; Energy transformation; Mitochondria.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

