Hepatic ketogenesis is not required for starvation adaptation in mice
- PMID: 38876267
- PMCID: PMC11246062
- DOI: 10.1016/j.molmet.2024.101967
Hepatic ketogenesis is not required for starvation adaptation in mice
Abstract
Objective: In response to bacterial inflammation, anorexia of acute illness is protective and is associated with the induction of fasting metabolic programs such as ketogenesis. Forced feeding during the anorectic period induced by bacterial inflammation is associated with suppressed ketogenesis and increased mortality. As ketogenesis is considered essential in fasting adaptation, we sought to determine the role of ketogenesis in illness-induced anorexia.
Methods: A mouse model of inducible hepatic specific deletion of the rate limiting enzyme for ketogenesis (HMG-CoA synthase 2, Hmgcs2) was used to investigate the role of ketogenesis in endotoxemia, a model of bacterial inflammation, and in prolonged starvation.
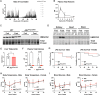
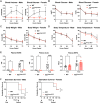
Results: Mice deficient of hepatic Hmgcs2 failed to develop ketosis during endotoxemia and during prolonged fasting. Surprisingly, hepatic HMGCS2 deficiency and the lack of ketosis did not affect survival, glycemia, or body temperature in response to endotoxemia. Mice with hepatic ketogenic deficiency also did not exhibit any defects in starvation adaptation and were able to maintain blood glucose, body temperature, and lean mass compared to littermate wild-type controls. Mice with hepatic HMGCS2 deficiency exhibited higher levels of plasma acetate levels in response to fasting.
Conclusions: Circulating hepatic-derived ketones do not provide protection against endotoxemia, suggesting that alternative mechanisms drive the increased mortality from forced feeding during illness-induced anorexia. Hepatic ketones are also dispensable for surviving prolonged starvation in the absence of inflammation. Our study challenges the notion that hepatic ketogenesis is required to maintain blood glucose and preserve lean mass during starvation, raising the possibility of extrahepatic ketogenesis and use of alternative fuels as potential means of metabolic compensation.
Keywords: Endotoxemia; Fasting metabolism; HMGCS2; Ketogenesis; Starvation adaptation.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Fasting-induced HMGCS2 expression in the kidney does not contribute to circulating ketones.Am J Physiol Renal Physiol. 2022 Apr 1;322(4):F460-F467. doi: 10.1152/ajprenal.00447.2021. Epub 2022 Feb 28. Am J Physiol Renal Physiol. 2022. PMID: 35224990 Free PMC article.
-
Role of ketone signaling in the hepatic response to fasting.Am J Physiol Gastrointest Liver Physiol. 2019 May 1;316(5):G623-G631. doi: 10.1152/ajpgi.00415.2017. Epub 2019 Feb 15. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30767679 Free PMC article.
-
Hmgcs2-mediated ketogenesis modulates high-fat diet-induced hepatosteatosis.Mol Metab. 2022 Jul;61:101494. doi: 10.1016/j.molmet.2022.101494. Epub 2022 Apr 12. Mol Metab. 2022. PMID: 35421611 Free PMC article.
-
Starvation Ketosis and the Kidney.Am J Nephrol. 2021;52(6):467-478. doi: 10.1159/000517305. Epub 2021 Jul 19. Am J Nephrol. 2021. PMID: 34350876 Review.
-
Hepatic metabolism and ketone production in metabolic dysfunction-associated steatotic liver disease.Curr Opin Gastroenterol. 2025 Mar 1;41(2):81-86. doi: 10.1097/MOG.0000000000001079. Epub 2025 Jan 2. Curr Opin Gastroenterol. 2025. PMID: 39782299 Review.
Cited by
-
Ketogenesis is dispensable for the metabolic adaptations to caloric restriction.bioRxiv [Preprint]. 2025 Jun 1:2025.05.29.656153. doi: 10.1101/2025.05.29.656153. bioRxiv. 2025. PMID: 40501543 Free PMC article. Preprint.
-
Ketogenesis mitigates metabolic dysfunction-associated steatotic liver disease through mechanisms that extend beyond fat oxidation.J Clin Invest. 2025 Apr 24;135(12):e191021. doi: 10.1172/JCI191021. eCollection 2025 Jun 16. J Clin Invest. 2025. PMID: 40272888 Free PMC article.
-
Ketone body mediated histone β-hydroxybutyrylation is reno-protective.bioRxiv [Preprint]. 2025 May 4:2024.12.18.628574. doi: 10.1101/2024.12.18.628574. bioRxiv. 2025. PMID: 39763881 Free PMC article. Preprint.
References
-
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

