The proteostasis interactomes of trafficking-deficient variants of the voltage-gated potassium channel KV11.1 associated with long QT syndrome
- PMID: 38876300
- PMCID: PMC11284683
- DOI: 10.1016/j.jbc.2024.107465
The proteostasis interactomes of trafficking-deficient variants of the voltage-gated potassium channel KV11.1 associated with long QT syndrome
Abstract
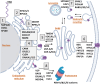
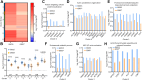
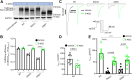
The voltage-gated potassium ion channel KV11.1 plays a critical role in cardiac repolarization. Genetic variants that render Kv11.1 dysfunctional cause long QT syndrome (LQTS), which is associated with fatal arrhythmias. Approximately 90% of LQTS-associated variants cause intracellular protein transport (trafficking) dysfunction, which pharmacological chaperones like E-4031 can rescue. Protein folding and trafficking decisions are regulated by chaperones, protein quality control factors, and trafficking machinery comprising the cellular proteostasis network. Here, we test whether trafficking dysfunction is associated with alterations in the proteostasis network of pathogenic Kv11.1 variants and whether pharmacological chaperones can normalize the proteostasis network of responsive variants. We used affinity-purification coupled with tandem mass tag-based quantitative mass spectrometry to assess protein interaction changes of WT KV11.1 or trafficking-deficient channel variants in the presence or absence of E-4031. We identified 572 core KV11.1 protein interactors. Trafficking-deficient variants KV11.1-G601S and KV11.1-G601S-G965∗ had significantly increased interactions with proteins responsible for folding, trafficking, and degradation compared to WT. We confirmed previous findings that the proteasome is critical for KV11.1 degradation. Our report provides the first comprehensive characterization of protein quality control mechanisms of KV11.1. We find extensive interactome remodeling associated with trafficking-deficient KV11.1 variants and with pharmacological chaperone rescue of KV11.1 cell surface expression. The identified protein interactions could be targeted therapeutically to improve KV11.1 trafficking and treat LQTS.
Keywords: hERG; interactome; ion channel; long QT syndrome; mass spectrometry; trafficking.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Update of
-
The proteostasis interactomes of trafficking-deficient K V 11.1 variants associated with Long QT Syndrome and pharmacological chaperone rescue.bioRxiv [Preprint]. 2024 Jan 31:2024.01.31.574410. doi: 10.1101/2024.01.31.574410. bioRxiv. 2024. Update in: J Biol Chem. 2024 Jul;300(7):107465. doi: 10.1016/j.jbc.2024.107465. PMID: 38352392 Free PMC article. Updated. Preprint.
Similar articles
-
The proteostasis interactomes of trafficking-deficient K V 11.1 variants associated with Long QT Syndrome and pharmacological chaperone rescue.bioRxiv [Preprint]. 2024 Jan 31:2024.01.31.574410. doi: 10.1101/2024.01.31.574410. bioRxiv. 2024. Update in: J Biol Chem. 2024 Jul;300(7):107465. doi: 10.1016/j.jbc.2024.107465. PMID: 38352392 Free PMC article. Updated. Preprint.
-
A High-Throughput Screening Assay to Identify Drugs that Can Treat Long QT Syndrome Caused by Trafficking-Deficient KV11.1 (hERG) Variants.Mol Pharmacol. 2022 Apr;101(4):236-245. doi: 10.1124/molpharm.121.000421. Epub 2022 Feb 6. Mol Pharmacol. 2022. PMID: 35125346 Free PMC article.
-
Pharmacological correction of long QT-linked mutations in KCNH2 (hERG) increases the trafficking of Kv11.1 channels stored in the transitional endoplasmic reticulum.Am J Physiol Cell Physiol. 2013 Nov 1;305(9):C919-30. doi: 10.1152/ajpcell.00406.2012. Epub 2013 Jul 17. Am J Physiol Cell Physiol. 2013. PMID: 23864605 Free PMC article.
-
Long QT Syndrome Type 2: Emerging Strategies for Correcting Class 2 KCNH2 (hERG) Mutations and Identifying New Patients.Biomolecules. 2020 Aug 4;10(8):1144. doi: 10.3390/biom10081144. Biomolecules. 2020. PMID: 32759882 Free PMC article. Review.
-
hERG K(+) channels: structure, function, and clinical significance.Physiol Rev. 2012 Jul;92(3):1393-478. doi: 10.1152/physrev.00036.2011. Physiol Rev. 2012. PMID: 22988594 Review.
References
-
- Bos J.M., Crotti L., Rohatgi R.K., Castelletti S., Dagradi F., Schwartz P.J., et al. Mexiletine shortens the QT interval in patients with potassium channel-mediated type 2 long QT syndrome. Circ. Arrhythm. Electrophysiol. 2019;12 - PubMed
-
- Sanguinetti M.C., Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

