Loss-of-function G6PD variant moderated high-fat diet-induced obesity, adipocyte hypertrophy, and fatty liver in male rats
- PMID: 38876306
- PMCID: PMC11328872
- DOI: 10.1016/j.jbc.2024.107460
Loss-of-function G6PD variant moderated high-fat diet-induced obesity, adipocyte hypertrophy, and fatty liver in male rats
Abstract
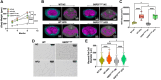
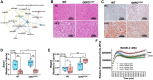
Obesity is a major risk factor for liver and cardiovascular diseases. However, obesity-driven mechanisms that contribute to the pathogenesis of multiple organ diseases are still obscure and treatment is inadequate. We hypothesized that increased , glucose-6-phosphate dehydrogenase (G6PD), the key rate-limiting enzyme in the pentose shunt, is critical in evoking metabolic reprogramming in multiple organs and is a significant contributor to the pathogenesis of liver and cardiovascular diseases. G6PD is induced by a carbohydrate-rich diet and insulin. Long-term (8 months) high-fat diet (HFD) feeding increased body weight and elicited metabolic reprogramming in visceral fat, liver, and aorta, of the wild-type rats. In addition, HFD increased inflammatory chemokines in visceral fat. Interestingly, CRISPR-edited loss-of-function Mediterranean G6PD variant (G6PDS188F) rats, which mimic human polymorphism, moderated HFD-induced weight gain and metabolic reprogramming in visceral fat, liver, and aorta. The G6PDS188F variant prevented HFD-induced CCL7 and adipocyte hypertrophy. Furthermore, the G6PDS188F variant increased Magel2 - a gene encoding circadian clock-related protein that suppresses obesity associated with Prader-Willi syndrome - and reduced HFD-induced non-alcoholic fatty liver. Additionally, the G6PDS188F variant reduced aging-induced aortic stiffening. Our findings suggest G6PD is a regulator of HFD-induced obesity, adipocyte hypertrophy, and fatty liver.
Keywords: chemokines; cytokines; fat tissue; inflammation; inter-organ communication; liver; metabolic reprogramming; vascular biology.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Wang Y., Chen X., Song Y., Caballero B., Cheskin L.J. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

