MeCP2 gene therapy ameliorates disease phenotype in mouse model for Pitt Hopkins syndrome
- PMID: 38876822
- PMCID: PMC11579869
- DOI: 10.1016/j.neurot.2024.e00376
MeCP2 gene therapy ameliorates disease phenotype in mouse model for Pitt Hopkins syndrome
Abstract
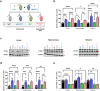
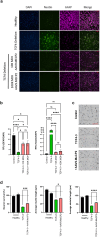
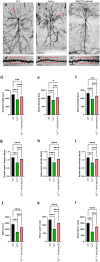
The neurodevelopmental disorder Pitt Hopkins syndrome (PTHS) causes clinical symptoms similar to Rett syndrome (RTT) patients. However, RTT is caused by MECP2 mutations whereas mutations in the TCF4 gene lead to PTHS. The mechanistic commonalities underling these two disorders are unknown, but their shared symptomology suggest that convergent pathway-level disruption likely exists. We reprogrammed patient skin derived fibroblasts into induced neuronal progenitor cells. Interestingly, we discovered that MeCP2 levels were decreased in PTHS patient iNPCs relative to healthy controls and that both iNPCs and iAstrocytes displayed defects in function and differentiation in a mutation-specific manner. When Tcf4+/- mice were genetically crossed with mice overexpressing MeCP2, molecular and phenotypic defects were significantly ameliorated, underlining and important role of MeCP2 in PTHS pathology. Importantly, post-natal intracerebroventricular gene replacement therapy with adeno-associated viral vector serotype 9 (AAV9)-expressing MeCP2 (AAV9.P546.MeCP2) significantly improved iNPC and iAstrocyte function and effectively ameliorated histological and behavioral defects in Tcf4+/- mice. Combined, our data suggest a previously unknown role of MeCP2 in PTHS pathology and common pathways that might be affected in multiple neurodevelopmental disorders. Our work highlights potential novel therapeutic targets for PTHS, including upregulation of MeCP2 expression or its downstream targets or, potentially, MeCP2-based gene therapy.
Keywords: AAV gene therapy; Mecp2; Pitt Hopkins syndrome; TCF4.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kathrin C Meyer, Patricia Cogram, Colleen M. Niswender reports financial support was provided by Pitt Hopkins Research Foundation. Colleen M. Niswender reports was provided by International Rett Syndrome Foundation. Rocco G. Gogliotti, Sheryl Anne D. Vermudez reports financial support was provided by National Institutes of Health. Kathrin Meyer, Cassandra Dennys has patent pending to Nationwide Children's Hospital. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Sweetser D.A., Elsharkawi I., Yonker L., Steeves M., Parkin K., Thibert R. In: GeneReviews® [Internet] Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., editors. University of Washington, Seattle; Seattle (WA): 2012 Aug 30. Pitt-hopkins syndrome.https://www.ncbi.nlm.nih.gov/books/NBK100240/ [Updated 2018 Apr 12] 1993-2024. Available from: - PubMed
-
- Giurgea I., Missirian C., Cacciagli P., Whalen S., Fredriksen T., Gaillon T., et al. TCF4 deletions in Pitt-Hopkins syndrome. Hum Mutat. 2008 Nov;29(11) - PubMed
-
- Dong J.Y., Fan P.D., Frizzell R.A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther [Internet] 1996 Nov 10;7(17):2101–2112. https://pubmed.ncbi.nlm.nih.gov/8934224/ [cited 2023 Jun 21] Available from: - PubMed
-
- Amir R.E., Van Den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl- CpG-binding protein 2. Nat Genet. 1999 Oct;23(2):185–188. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

