Mastoparan-7 adjuvanted COBRA H1 and H3 hemagglutinin influenza vaccines
- PMID: 38877101
- PMCID: PMC11178843
- DOI: 10.1038/s41598-024-64351-7
Mastoparan-7 adjuvanted COBRA H1 and H3 hemagglutinin influenza vaccines
Abstract
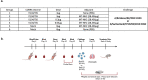
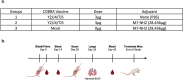
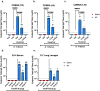
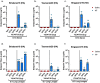
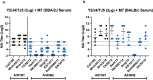
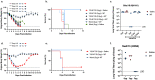
Adjuvants enhance, prolong, and modulate immune responses by vaccine antigens to maximize protective immunity and enable more effective immunization in the young and elderly. Most adjuvants are formulated with injectable vaccines. However, an intranasal route of vaccination may induce mucosal and systemic immune responses for enhancing protective immunity in individuals and be easier to administer compared to injectable vaccines. In this study, a next generation of broadly-reactive influenza hemagglutinin (HA) vaccines were developed using the Computationally Optimized Broadly Reactive Antigen (COBRA) methodology. These HA vaccines were formulated with Mastoparan 7 (M7-NH2) mast cell degranulating peptide adjuvant and administered intranasally to determine vaccine-induced seroconversion of antibodies against a panel of influenza viruses and protection following infection with H1N1 and H3N2 viruses in mice. Mice vaccinated intranasally with M7-NH2-adjuvanted COBRA HA vaccines had high HAIs against a panel of H1N1 and H3N2 influenza viruses and were protected against both morbidity and mortality, with reduced viral lung titers, following challenge with an H1N1 influenza virus. Additionally, M7-NH2 adjuvanted COBRA HA vaccines induced Th2 skewed immune responses with robust IgG and isotype antibodies in the serum and mucosal lung lavages. Overall, this intranasally delivered M7-NH2 -adjuvanted COBRA HA vaccine provides effective protection against drifted H1N1 and H3N2 viruses.
Keywords: Adjuvant; COBRA; Influenza; Mastoparan; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Bivalent H1 and H3 COBRA Recombinant Hemagglutinin Vaccines Elicit Seroprotective Antibodies against H1N1 and H3N2 Influenza Viruses from 2009 to 2019.J Virol. 2022 Apr 13;96(7):e0165221. doi: 10.1128/jvi.01652-21. Epub 2022 Mar 15. J Virol. 2022. PMID: 35289635 Free PMC article.
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
A single dose of inactivated influenza virus vaccine expressing COBRA hemagglutinin elicits broadly-reactive and long-lasting protection.PLoS One. 2025 Feb 21;20(2):e0308680. doi: 10.1371/journal.pone.0308680. eCollection 2025. PLoS One. 2025. PMID: 39982912 Free PMC article.
-
Mast cell activators as adjuvants for intranasal mucosal vaccines.Int J Pharm. 2025 Mar 15;672:125300. doi: 10.1016/j.ijpharm.2025.125300. Epub 2025 Feb 4. Int J Pharm. 2025. PMID: 39914508 Review.
-
Adjuvantation of Influenza Vaccines to Induce Cross-Protective Immunity.Vaccines (Basel). 2021 Jan 21;9(2):75. doi: 10.3390/vaccines9020075. Vaccines (Basel). 2021. PMID: 33494477 Free PMC article. Review.
Cited by
-
Nasal immunization with compound 48/80-adjuvanted acellular pertussis vaccines is an effective strategy to induce pertussis-specific systemic and mucosal immunity.Clin Exp Vaccine Res. 2025 Jul;14(3):246-260. doi: 10.7774/cevr.2025.14.e23. Epub 2025 Apr 15. Clin Exp Vaccine Res. 2025. PMID: 40741062 Free PMC article.
-
The Development of a Novel Broad-Spectrum Influenza Polypeptide Vaccine Based on Multi-Epitope Tandem Sequences.Vaccines (Basel). 2025 Jan 17;13(1):81. doi: 10.3390/vaccines13010081. Vaccines (Basel). 2025. PMID: 39852860 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

