Genome-wide analyses of member identification, expression pattern, and protein-protein interaction of EPF/EPFL gene family in Gossypium
- PMID: 38877405
- PMCID: PMC11177404
- DOI: 10.1186/s12870-024-05262-7
Genome-wide analyses of member identification, expression pattern, and protein-protein interaction of EPF/EPFL gene family in Gossypium
Abstract
Background: Epidermal patterning factor / -like (EPF/EPFL) gene family encodes a class of cysteine-rich secretory peptides, which are widelyfound in terrestrial plants.Multiple studies has indicated that EPF/EPFLs might play significant roles in coordinating plant development and growth, especially as the morphogenesis processes of stoma, awn, stamen, and fruit skin. However, few research on EPF/EPFL gene family was reported in Gossypium.
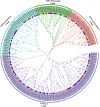
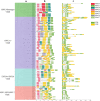
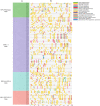
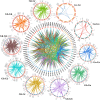
Results: We separately identified 20 G. raimondii, 24 G. arboreum, 44 G. hirsutum, and 44 G. barbadense EPF/EPFL genes in the 4 representative cotton species, which were divided into four clades together with 11 Arabidopsis thaliana, 13 Oryza sativa, and 17 Selaginella moellendorffii ones based on their evolutionary relationships. The similar gene structure and common motifs indicated the high conservation among the EPF/EPFL members, while the uneven distribution in chromosomes implied the variability during the long-term evolutionary process. Hundreds of collinearity relationships were identified from the pairwise comparisons of intraspecifc and interspecific genomes, which illustrated gene duplication might contribute to the expansion of cotton EPF/EPFL gene family. A total of 15 kinds of cis-regulatory elements were predicted in the promoter regions, and divided into three major categories relevant to the biological processes of development and growth, plant hormone response, and abiotic stress response. Having performing the expression pattern analyses with the basic of the published RNA-seq data, we found most of GhEPF/EPFL and GbEPF/EPFL genes presented the relatively low expression levels among the 9 tissues or organs, while showed more dramatically different responses to high/low temperature and salt or drought stresses. Combined with transcriptome data of developing ovules and fibers and quantitative Real-time PCR results (qRT-PCR) of 15 highly expressed GhEPF/EPFL genes, it could be deduced that the cotton EPF/EPFL genes were closely related with fiber development. Additionally, the networks of protein-protein interacting among EPF/EPFLs concentrated on the cores of GhEPF1 and GhEPF7, and thosefunctional enrichment analyses indicated that most of EPF/EPFLs participate in the GO (Gene Ontology) terms of stomatal development and plant epidermis development, and the KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways of DNA or base excision repair.
Conclusion: Totally, 132 EPF/EPFL genes were identified for the first time in cotton, whose bioinformatic analyses of cis-regulatory elements and expression patterns combined with qRT-PCR experiments to prove the potential functions in the biological processes of plant growth and responding to abiotic stresses, specifically in the fiber development. These results not only provide comprehensive and valuable information for cotton EPF/EPFL gene family, but also lay solid foundation for screening candidate EPF/EPFL genes in further cotton breeding.
Keywords: EPF/EPFL gene family; Cotton; Expression pattern; Protein–protein interaction; qRT-PCR verification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Genome-wide identification and expression-pattern analysis of sulfate transporter (SULTR) gene family in cotton under multiple abiotic stresses and fiber development.Funct Integr Genomics. 2024 May 22;24(3):108. doi: 10.1007/s10142-024-01387-y. Funct Integr Genomics. 2024. PMID: 38773054
-
Comprehensive identification of cotton EPF/EPFL receptors and functional characterization of the GhEPFL1-1-GhER1 module in drought tolerance.BMC Plant Biol. 2025 Jul 11;25(1):901. doi: 10.1186/s12870-025-06797-z. BMC Plant Biol. 2025. PMID: 40646466 Free PMC article.
-
Genome-wide identification and phylogenetic and expression pattern analyses of EPF/EPFL family genes in the Rye (Secale cereale L.).BMC Genomics. 2024 May 30;25(1):532. doi: 10.1186/s12864-024-10425-9. BMC Genomics. 2024. PMID: 38816796 Free PMC article.
-
Genome-Wide Analysis of the NF-YB Gene Family in Gossypium hirsutum L. and Characterization of the Role of GhDNF-YB22 in Embryogenesis.Int J Mol Sci. 2018 Feb 6;19(2):483. doi: 10.3390/ijms19020483. Int J Mol Sci. 2018. PMID: 29415481 Free PMC article.
-
Genome-wide expression analysis of phospholipase A1 (PLA1) gene family suggests phospholipase A1-32 gene responding to abiotic stresses in cotton.Int J Biol Macromol. 2021 Dec 1;192:1058-1074. doi: 10.1016/j.ijbiomac.2021.10.038. Epub 2021 Oct 14. Int J Biol Macromol. 2021. PMID: 34656543 Review.
Cited by
-
Genome wide identification and characterization of EPFL genes and its potential association with male sterility in Brassica rapa.Sci Rep. 2025 Jul 5;15(1):24033. doi: 10.1038/s41598-025-09555-1. Sci Rep. 2025. PMID: 40617881 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

