Dysbiosis in pregnant mice induced by transfer of human vaginal microbiota followed by reversal of pathological changes in the uterus and placenta via progesterone treatment
- PMID: 38877443
- PMCID: PMC11177491
- DOI: 10.1186/s12884-024-06595-9
Dysbiosis in pregnant mice induced by transfer of human vaginal microbiota followed by reversal of pathological changes in the uterus and placenta via progesterone treatment
Abstract
Objective: The vaginal microbiota dysbiosis induces inflammation in the uterus that triggers tissue damage and is associated with preterm birth. Progesterone is used to prevent labor in pregnant women at risk of preterm birth. However, the mechanism of action of progesterone still needs to be clarified. We aimed to show the immunomodulatory effect of progesterone on the inflammation of uterine tissue triggered by dysbiotic vaginal microbiota in a pregnant mouse model.
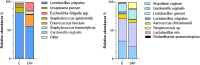
Methods: Healthy (n = 6) and dysbiotic (n = 7) vaginal microbiota samples isolated from pregnant women were transferred to control (n = 10) and dysbiotic (n = 14) pregnant mouse groups. The dysbiotic microbiota transferred group was treated with 1 mg progesterone (n = 7). Flow cytometry and immunohistochemistry analyses were used to evaluate inflammatory processes. Vaginal microbiota samples were analyzed by 16 S rRNA sequencing.
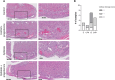
Results: Vaginal exposure to dysbiotic microbiota resulted in macrophage accumulation in the uterus and cellular damage in the placenta. Even though TNF and IL-6 elevations were not significant after dysbiotic microbiota transplantation, progesterone treatment decreased TNF and IL-6 expressions from 49.085 to 31.274% (p = 0.0313) and 29.279-21.216% (p = 0.0167), respectively. Besides, the macrophage density in the uterus was reduced, and less cellular damage in the placenta was observed.
Conclusion: Analyzing the vaginal microbiota before or during pregnancy may support the decision for initiation of progesterone therapy. Our results also guide the development of new strategies for preventing preterm birth.
Keywords: Animal model; Dysbiosis; Inflammation; Pregnancy; Preterm birth; Progesterone; Vaginal microbiota.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, Rao AV, McNellis D. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. - DOI - PubMed
-
- Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, Van Lierde S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009;116(10):1315–1324. doi: 10.1111/j.1471-0528.2009.02237.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

