Determination of levofloxacin, norfloxacin, and moxifloxacin in pharmaceutical dosage form or individually using derivative UV spectrophotometry
- PMID: 38877570
- PMCID: PMC11179347
- DOI: 10.1186/s13065-024-01193-4
Determination of levofloxacin, norfloxacin, and moxifloxacin in pharmaceutical dosage form or individually using derivative UV spectrophotometry
Abstract
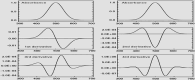
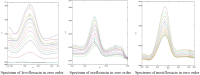
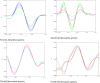
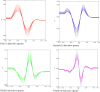
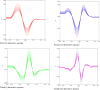
Purpose: In this study, first, second, third, and fourth-order derivative spectrophotometric methods utilizing the peak-zero (P-O) and peak-peak (P-P) techniques of measurement were developed for the determination of levofloxacin, norfloxacin, and moxifloxacin. These methods were applied to their combined pharmaceutical dosage form or individually for levofloxacin, norfloxacin, and moxifloxacin.
Methods: Linearity was established in the concentration range of 2-20 µg/mL. The procedures are simple, quick, and precise. The developed methods are sensitive, accurate, and cost-effective, demonstrating excellent correlation coefficients (R2 = 0.9998) and mean recovery values ranging from 99.20% to 100.08%, indicating a high level of precision.
Results: The developed approach was effectively employed to determine the levofloxacin, norfloxacin, and moxifloxacin content in commercially available pharmaceutical dosages.
Conclusions: Statistical analysis and recovery tests confirmed the method's linearity and accuracy. The results suggest that this method can be utilized for routine analysis in both bulk and commercial formulations. The simplicity, accuracy, and cost-effectiveness of the developed methods make them valuable for pharmaceutical analysis.
Keywords: Derivative Uv spectrophotometry; Levofloxacin; Moxifloxacin; Norfloxacin; Simultaneous estimation; Validation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Redasani VK, Patel PR, Marathe DY, Chaudhari SR, Shirkhedkar AA, Surana SJ. A review on derivative UV-spectrophotometry analysis of drugs in pharmaceutical formulations and biological samples. J Chil Chem Soc. 2018;63(3):4126–4134. doi: 10.4067/s0717-97072018000304126. - DOI
-
- Ojeda CB, Rojas FS. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Anal Chim Acta. 2004;518(1–2):1–24. doi: 10.1016/j.aca.2004.04.031. - DOI
-
- Karpinska, J. Basic principles and analytical application of derivative spectrophotometry. Macro to nano spectroscopy, Macro to nano spectroscopy, book edited by Jamal Uddin (2012):253–256.
LinkOut - more resources
Full Text Sources
Miscellaneous
