Heavy metals in cigarette smoke strongly inhibit pancreatic ductal function and promote development of chronic pancreatitis
- PMID: 38877637
- PMCID: PMC11178517
- DOI: 10.1002/ctm2.1733
Heavy metals in cigarette smoke strongly inhibit pancreatic ductal function and promote development of chronic pancreatitis
Abstract
Background and aims: Smoking is recognised as an independent risk factor in the development of chronic pancreatitis (CP). Cystic fibrosis transmembrane conductance regulator (CFTR) function and ductal fluid and bicarbonate secretion are also known to be impaired in CP, so it is crucial to understand the relationships between smoking, pancreatic ductal function and the development of CP.
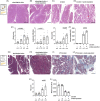
Methods: We measured sweat chloride (Cl-) concentrations in patients with and without CP, both smokers and non-smokers, to assess CFTR activity. Serum heavy metal levels and tissue cadmium concentrations were determined by mass spectrometry in smoking and non-smoking patients. Guinea pigs were exposed to cigarette smoke, and cigarette smoke extract (CSE) was prepared to characterise its effects on pancreatic HCO3 - and fluid secretion and CFTR function. We administered cerulein to both the smoking and non-smoking groups of mice to induce pancreatitis.
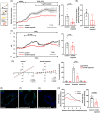
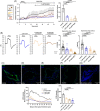
Results: Sweat samples from smokers, both with and without CP, exhibited elevated Cl- concentrations compared to those from non-smokers, indicating a decrease in CFTR activity due to smoking. Pancreatic tissues from smokers, regardless of CP status, displayed lower CFTR expression than those from non-smokers. Serum levels of cadmium and mercury, as well as pancreatic tissue cadmium, were increased in smokers. Smoking, CSE, cadmium, mercury and nicotine all hindered fluid and HCO3 - secretion and CFTR activity in pancreatic ductal cells. These effects were mediated by sustained increases in intracellular calcium ([Ca2+]i), depletion of intracellular ATP (ATPi) and mitochondrial membrane depolarisation.
Conclusion: Smoking impairs pancreatic ductal function and contributes to the development of CP. Heavy metals, notably cadmium, play a significant role in the harmful effects of smoking.
Key points: Smoking and cigarette smoke extract diminish pancreatic ductal fluid and HCO3 - secretion as well as the expression and function of CFTR Cd and Hg concentrations are significantly higher in the serum samples of smokers Cd accumulates in the pancreatic tissue of smokers.
Keywords: CFTR; cadmium; chronic pancreatitis; epithelial ion secretion; smoking.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors have no relevant financial or non‐financial interests to disclose.
Figures



References
-
- Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184‐1197. - PubMed
-
- Tattersall SJN, Apte MV, Wilson JS. A fire inside: current concepts in chronic pancreatitis. Intern Med J. 2008;38:592‐598. - PubMed
-
- Kleeff J, Whitcomb DC, Shimosegawa T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. - PubMed
-
- Olesen SS, Phillips AE, Faghih M, et al. Overlap and cumulative effects of pancreatic duct obstruction, abnormal pain processing and psychological distress on patient‐reported outcomes in chronic pancreatitis. Gut. 2022;71:2518‐2525. - PubMed
-
- Jagannath S, Garg PK. Novel and experimental therapies in chronic pancreatitis. Dig Dis Sci. 2017;62:1751‐1761. - PubMed
MeSH terms
Substances
Grants and funding
- K131996/Hungarian National Research, Development and Innovation Office
- K120335/Hungarian National Research, Development and Innovation Office
- PD116553/Hungarian National Research, Development and Innovation Office
- FK139269/Hungarian National Research, Development and Innovation Office
- LP2017-18/2017/Hungarian Academy of Science
- LP2014-10/2014/Hungarian Academy of Science
- BO/00598/19/5/Hungarian Academy of Science
- TKP2021-EGA-23/Hungarian Ministry of Innovation and Technology from the Hungarian National Research, Development and Innovation Fund
- TKP2021-EGA-28/Hungarian Ministry of Innovation and Technology from the Hungarian National Research, Development and Innovation Fund
- TKP2021-EGA-13/Hungarian Ministry of Innovation and Technology from the Hungarian National Research, Development and Innovation Fund
- TKP2021-NVA-19/Hungarian Ministry of Innovation and Technology from the Hungarian National Research, Development and Innovation Fund
- 739593/European Union's Horizon 2020 Research and Innovation Program
- RRF-2.3.1-21-2022-00011/Translational Neuroscience National Laboratory Program
- RRF-2.3.1-21-2002-00015/National Laboratory of Drug Research and Development
LinkOut - more resources
Full Text Sources
Miscellaneous
