Impact of trypsin on cell cytoplasm during detachment of cells studied by terahertz sensing
- PMID: 38877703
- PMCID: PMC11365101
- DOI: 10.1016/j.bpj.2024.06.011
Impact of trypsin on cell cytoplasm during detachment of cells studied by terahertz sensing
Abstract
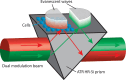
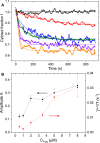
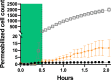
Trypsin is a very common enzyme used in cell culture to harvest cells by cleaving the proteins responsible for cell adhesion. However, trypsin also induces undesirable effects on cells, such as altering membrane proteins and the cytoskeleton, changing the composition of the cytoplasm and the cell volume, and even leading to cell death when used improperly. Using attenuated total reflection in the terahertz domain, confocal microscopy, and the propidium iodide test, we quantified in real time the change in cytoplasmic content induced by trypsin proteolysis on Madin-Darby canine kidney epithelial cells. We have observed a cytoplasmic modification from the very first seconds of trypsinization, following the change of cell volume due to mechanical re-equilibrium of the membrane. We found that the cytoplasmic alteration is associated with a transfer of small solutes: electrolytes and metabolites. We also found a very good nonlinear correlation between the side effects monitored by terahertz sensing and the cell height, regardless of the dependence of the cell height on trypsin concentration and exposure time.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Campbell N. 11th edition. Pearson; 2016. Biology.
-
- Fischer B., Meier A., et al. Gentile L. A Complete Workflow for the Differentiation and the Dissociation of hiPSC-derived Cardiospheres. Stem Cell Res. 2018;32:65–72. - PubMed
-
- Harrison C.J., Allen T.D. Cell Surface Morphology After Trypsinisation Depends on Initial Cell Shape. Differentiation. 1979;15:61–66. - PubMed
-
- Furcht L.T., Wendelschafer-Crabb G. Trypsin-Induced Coordinate Alterations in Cell Shape, Cytoskeleton, and Intrinsic Membrane Structure of Contact-Inhibited Cells. Exp. Cell Res. 1978;114:1–14. - PubMed
-
- Yan J., Xie C., et al. Li L. Effect of Trypsin Concentration on Living SMCC-7721 Cells Studied by Atomic Force Microscopy. J. Microsc. 2021;284:203–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

