Alternative magnetic field exposure suppresses tumor growth via metabolic reprogramming
- PMID: 38877783
- PMCID: PMC11309929
- DOI: 10.1111/cas.16243
Alternative magnetic field exposure suppresses tumor growth via metabolic reprogramming
Abstract
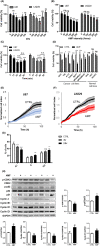
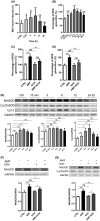
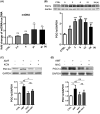
Application of physical forces, ranging from ultrasound to electric fields, is recommended in various clinical practice guidelines, including those for treating cancers and bone fractures. However, the mechanistic details of such treatments are often inadequately understood, primarily due to the absence of comprehensive study models. In this study, we demonstrate that an alternating magnetic field (AMF) inherently possesses a direct anti-cancer effect by enhancing oxidative phosphorylation (OXPHOS) and thereby inducing metabolic reprogramming. We observed that the proliferation of human glioblastoma multiforme (GBM) cells (U87 and LN229) was inhibited upon exposure to AMF within a specific narrow frequency range, including around 227 kHz. In contrast, this exposure did not affect normal human astrocytes (NHA). Additionally, in mouse models implanted with human GBM cells in the brain, daily exposure to AMF for 30 min over 21 days significantly suppressed tumor growth and prolonged overall survival. This effect was associated with heightened reactive oxygen species (ROS) production and increased manganese superoxide dismutase (MnSOD) expression. The anti-cancer efficacy of AMF was diminished by either a mitochondrial complex IV inhibitor or a ROS scavenger. Along with these observations, there was a decrease in the extracellular acidification rate (ECAR) and an increase in the oxygen consumption rate (OCR). This suggests that AMF-induced metabolic reprogramming occurs in GBM cells but not in normal cells. Our results suggest that AMF exposure may offer a straightforward strategy to inhibit cancer cell growth by leveraging oxidative stress through metabolic reprogramming.
Keywords: alternating magnetic field (AMF); glioblastoma multiforme (GBM); metabolic reprogramming; mitochondria; oxidative phosphorylation (OXPHOS); reactive oxygen species (ROS).
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
We have received funding for a portion of our research from Ricoh Company, Ltd.
Figures




References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492‐507. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. - PubMed
-
- Blumberger DM, Vila‐Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high‐frequency repetitive transcranial magnetic stimulation in patients with depression (THREE‐D): a randomised non‐inferiority trial. Lancet. 2018;391:1683‐1692. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

