Impact of Prior Biologic or Janus Kinase Inhibitor Therapy on Efficacy and Safety of Etrasimod in the ELEVATE UC 52 and ELEVATE UC 12 Trials
- PMID: 38877972
- PMCID: PMC11532610
- DOI: 10.1093/ecco-jcc/jjae079
Impact of Prior Biologic or Janus Kinase Inhibitor Therapy on Efficacy and Safety of Etrasimod in the ELEVATE UC 52 and ELEVATE UC 12 Trials
Erratum in
-
Corrigendum to: Impact of Prior Biologic or Janus Kinase Inhibitor Therapy on Efficacy and Safety of Etrasimod in the ELEVATE UC 52 and ELEVATE UC 12 Trials.J Crohns Colitis. 2024 Nov 4;18(11):1935-1936. doi: 10.1093/ecco-jcc/jjae156. J Crohns Colitis. 2024. PMID: 39366012 Free PMC article. No abstract available.
Abstract
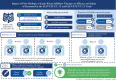
Background and aims: Etrasimod is an oral, once daily, selective, sphingosine 1-phosphate [S1P]1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis [UC]. This subgroup analysis evaluated the efficacy and safety of etrasimod 2 mg once daily vs placebo by prior biologic/Janus kinase inhibitor [bio/JAKi] exposure in ELEVATE UC 52 and ELEVATE UC 12.
Methods: Pre-defined efficacy endpoints were assessed at Weeks 12 and 52 in ELEVATE UC 52 and Week 12 in ELEVATE UC 12 in bio/JAKi-naïve and -experienced patients, and at Week 12 [pooled] based on prior advanced therapy exposure mechanism.
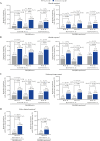
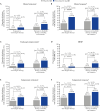
Results: In the ELEVATE UC 52 and ELEVATE UC 12 analysis populations, 80/274 [29.2%] and 74/222 [33.3%] patients receiving etrasimod and 42/135 [31.1%] and 38/112 [33.9%] patients receiving placebo, respectively, were bio/JAKi-experienced. In both bio/JAKi-naïve and -experienced patients, a significantly greater proportion receiving etrasimod vs placebo achieved clinical remission [p < 0.05] in ELEVATE UC 52 at Weeks 12 [naïve: 30.9% vs 9.7%; experienced: 17.5% vs 2.4%] and 52 [naïve: 36.6% vs 7.5%; experienced: 21.3% vs 4.8%]; in ELEVATE UC 12, this was observed only for bio/JAKi-naïve patients [naïve: 27.7% vs 16.2%, p = 0.033; experienced: 18.9% vs 13.2%, p = 0.349]. Similar patterns were observed for most efficacy endpoints. Among patients with prior anti-integrin exposure [N = 90], a significantly greater proportion achieved clinical response [54.1% vs 27.6%, p = 0.030], but not clinical remission [9.8% vs 3.4%, p = 0.248], with etrasimod vs placebo.
Conclusions: Bio/JAKi-naïve and -experienced patients had clinically meaningful induction and maintenance treatment benefits with etrasimod vs placebo.
Clinicaltrials.gov: NCT03945188; NCT03996369.
Keywords: Ulcerative colitis; advanced therapies; etrasimod.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
SV received lecture/speaker fees from AbbVie, Dr. Falk Pharma, Ferring, Galapagos, Hospira, Janssen, MSD, Takeda, and Tillotts; consultancy/advisory fees from AbbVie, AbolerIS Pharma, Alimentiv, Arena, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr. Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead Sciences, Hospira, IMIDomics, Janssen, Johnson and Johnson, Materia Prima, MiroBio, Morphic, MRM Health, MSD, Mundipharma, Pfizer Inc, ProDigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance Biopharma, Tillotts, and Zealand Pharma; grants/research support from AbbVie, Pfizer Inc, Galapagos, Janssen, and Takeda. BES: received consulting fees from AbbVie, Abivax, Alimentiv, Amgen, Arena, Artugen Therapeutics, AstraZeneca, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Fresenius Kabi, Galapagos, Genentech [Roche], Gilead Sciences, GlaxoSmithKline, Gossamer Bio, InDex Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Morphic, MRM Health, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sun Pharma, Surrozen, Takeda, Target RWE, Teva, Theravance Biopharma, TLL Pharmaceutical, Ventyx Biosciences; speaking fees from Abivax, Bristol Myers Squibb, Janssen, Lilly, Pfizer, Takeda; research grants from Bristol Myers Squibb, Janssen, Pfizer, Takeda, Theravance Biopharma; other support from Bristol Myers Squibb, Janssen, Lilly, Pfizer, Takeda; shareholder/stock options for Ventyx Biosciences. LP-B received fees from Adacyte, AbbVie, Abivax, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Celltrion, Connect Biopharma, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, Gossamer Bio, GSK, HAC Pharma, IAG Image Analysis, InDex Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Nordic Pharma, Novartis, OM Pharma, ONO Pharmaceutical Co, Ose Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer Inc, Prometheus, Protagonist Therapeutics, Roche, Roivant Sciences, Samsung, Sandoz, Sanofi, Takeda, Theravance Biopharma, Thermo Fisher, TiGenix, Tillotts, Viatris, VectivBio, Ventyx, Vifor, and Ysopia. GRD’H served as an adviser for AbbVie, AstraZeneca, Alimentiv, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Cosmo, Eli Lilly, Galapagos, GSK, Johnson and Johnson, Takeda, Pfizer Inc, Polpharma, Prometheus Biosciences, Tillotts, and Ventyx; received speaker fees from AbbVie, Boehringer Ingelheim, Celltrion, Eli Lilly, Johnson and Johnson, Takeda, Pfizer Inc, and Tillotts. JP received personal fees from AbbVie, Arena, Athos, Atomwise, Boehringer Ingelheim, Celgene, Celsius, Celltrion, Ferring, Galapagos, Genentech/Roche, GSK, Janssen, Mirum, Morphic, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus, Protagonist Therapeutics, Revolo Biotherapeutics, Sanofi, Takeda, Theravance Biopharma, and Wassermann; grant support from AbbVie and Pfizer Inc. AJY received consultancy fees from Pfizer Inc, Arena, Takeda, and Bristol Myers Squibb; lecture and speaking fees from Bristol Myers Squibb. DCW received research grant support from AbbVie, Arena, Bristol Myers Squibb, Janssen, Pfizer Inc, Takeda, and Ventyx; lecture fees from AbbVie, Bristol Myers Squibb, Janssen, Pfizer Inc, and Takeda; consultancy fees from AbbVie, Arena, Bristol Myers Squibb, Janssen, Pfizer Inc, and Takeda. TR served on speaker panels for Takeda, Janssen, Pfizer Inc, Bristol Myers Squibb, AbbVie, and Lilly; served on a data adjudication committee for Ferring/Rebiotix; served on advisory boards for AbbVie, Ardelyx, Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ferring, Genentech/Roche, Gilead Sciences, and Intercept Pharma; shareholder for Iterative Scopes. SS received lecture/speaker fees from AbbVie, Arena, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Fresenius Kabi, Janssen, MSD, Pfizer Inc, and Takeda; consultancy/advisory fees from AbbVie, Arena, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Dr. Falk Pharma, Fresenius Kabi, Gilead Sciences, I-Mab, Janssen, MSD, Mylan, Pfizer Inc, Protagonist Therapeutics, Provention Bio, Takeda, and Theravance Biopharma. JW, IM, MK, KS, JW, and MG are employees and shareholders of Pfizer Inc. MVC received grant support from Pfizer Inc, Janssen, Novartis, and BMS; consulting fees from AbbVie, Arena, BMS, Medtronic, Pfizer Inc, Prometheus, Lilly, Janssen, and Takeda; speaker fees from AbbVie, Janssen, Medtronic, Pfizer Inc, BMS, Takeda, and Fresenius Kabi. FB received financial support for research/grants from AbbVie, Amgen, Janssen, and Takeda; received lecture fees and served on a speaker’s bureau for AbbVie, Arena, Celltrion, Ferring, Galapagos, Janssen, Merck Sharp & Dohme, Pfizer Inc, and Takeda; consultancy fees from AbbVie, Amgen, Arena, Celgene, Celltrion, Ferring, Fresenius Kabi, Janssen, Merck Sharp & Dohme, Pfizer Inc, and Sandoz. MCD received consulting fees from AbbVie, Abivax, Arena, AstraZeneca, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Genentech, Gilead Sciences, Janssen, Pfizer Inc, Prometheus Laboratories, Prometheus Biosciences, Takeda, and UCB; grant/research support from Janssen; shareholder/royalties and directorship/ownership interest in Trellus Health. SD received lecture/speaker fees from AbbVie, Amgen, Ferring, Gilead Sciences, Janssen, Mylan, Pfizer Inc, and Takeda; consulting/advisory fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead Sciences, Hospira, Janssen, Johnson and Johnson, MSD, Mundipharma, Pfizer Inc, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor; holds directorship/ownership in
Figures


References
-
- Magro F, Gionchetti P, Eliakim R, et al.; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. - PubMed
-
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. - PubMed
-
- Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis 2022;16:2–17. - PubMed
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.. ACG Clinical Guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

