Phospholipid scramblase 1: an essential component of the nephrocyte slit diaphragm
- PMID: 38878170
- PMCID: PMC11335299
- DOI: 10.1007/s00018-024-05287-z
Phospholipid scramblase 1: an essential component of the nephrocyte slit diaphragm
Abstract
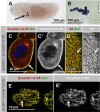
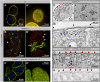
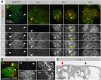
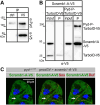
Blood ultrafiltration in nephrons critically depends on specialized intercellular junctions between podocytes, named slit diaphragms (SDs). Here, by studying a homologous structure found in Drosophila nephrocytes, we identify the phospholipid scramblase Scramb1 as an essential component of the SD, uncovering a novel link between membrane dynamics and SD formation. In scramb1 mutants, SDs fail to form. Instead, the SD components Sticks and stones/nephrin, Polychaetoid/ZO-1, and the Src-kinase Src64B/Fyn associate in cortical foci lacking the key SD protein Dumbfounded/NEPH1. Scramb1 interaction with Polychaetoid/ZO-1 and Flotillin2, the presence of essential putative palmitoylation sites and its capacity to oligomerize, suggest a function in promoting SD assembly within lipid raft microdomains. Furthermore, Scramb1 interactors as well as its functional sensitivity to temperature, suggest an active involvement in membrane remodeling processes during SD assembly. Remarkably, putative Ca2+-binding sites in Scramb1 are essential for its activity raising the possibility that Ca2+ signaling may control the assembly of SDs by impacting on Scramb1 activity.
Keywords: Intercellular junction; Membrane dynamics; Slit diaphragm.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms.J Cell Biol. 2019 Jul 1;218(7):2294-2308. doi: 10.1083/jcb.201810171. Epub 2019 Jun 6. J Cell Biol. 2019. PMID: 31171632 Free PMC article.
-
Src64B phosphorylates Dumbfounded and regulates slit diaphragm dynamics: Drosophila as a model to study nephropathies.Development. 2014 Jan;141(2):367-76. doi: 10.1242/dev.099408. Epub 2013 Dec 11. Development. 2014. PMID: 24335255
-
The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm.Nature. 2009 Jan 15;457(7227):322-6. doi: 10.1038/nature07526. Epub 2008 Oct 29. Nature. 2009. PMID: 18971929 Free PMC article.
-
Lipid-protein interactions along the slit diaphragm of podocytes.J Am Soc Nephrol. 2009 Mar;20(3):473-8. doi: 10.1681/ASN.2008070694. Epub 2009 Feb 25. J Am Soc Nephrol. 2009. PMID: 19244577 Review.
-
The long journey through renal filtration: new pieces in the puzzle of slit diaphragm architecture.Curr Opin Nephrol Hypertens. 2017 May;26(3):148-153. doi: 10.1097/MNH.0000000000000322. Curr Opin Nephrol Hypertens. 2017. PMID: 28212178 Review.
Cited by
-
Research progress in the treatment of lipid metabolism disorder in patients with diabetic kidney disease by the integrated traditional Chinese and Western medicine.Front Endocrinol (Lausanne). 2025 Jul 30;16:1631312. doi: 10.3389/fendo.2025.1631312. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40810065 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

