Population pharmacokinetics of TLD-1, a novel liposomal doxorubicin, in a phase I trial
- PMID: 38878207
- PMCID: PMC11420315
- DOI: 10.1007/s00280-024-04679-z
Population pharmacokinetics of TLD-1, a novel liposomal doxorubicin, in a phase I trial
Abstract
Study objectives: TLD-1 is a novel pegylated liposomal doxorubicin (PLD) formulation aiming to optimise the PLD efficacy-toxicity ratio. We aimed to characterise TLD-1's population pharmacokinetics using non-compartmental analysis and nonlinear mixed-effects modelling.
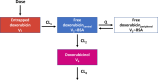
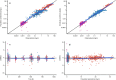
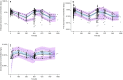
Methods: The PK of TLD-1 was analysed by performing a non-compartmental analysis of longitudinal doxorubicin plasma concentration measurements obtained from a clinical trial in 30 patients with advanced solid tumours across a 4.5-fold dose range. Furthermore, a joint parent-metabolite PK model of doxorubicinentrapped, doxorubicinfree, and metabolite doxorubicinol was developed. Interindividual and interoccasion variability around the typical PK parameters and potential covariates to explain parts of this variability were explored.
Results: Medians standard deviations of dose-normalised doxorubicinentrapped+free Cmax and AUC0-∞ were 0.342 0.134 mg/L and 40.1 18.9 mg·h/L, respectively. The median half-life (95 h) was 23.5 h longer than the half-life of currently marketed PLD. The novel joint parent-metabolite model comprised a one-compartment model with linear release (doxorubicinentrapped), a two-compartment model with linear elimination (doxorubicinfree), and a one-compartment model with linear elimination for doxorubicinol. Body surface area on the volumes of distribution for free doxorubicin was the only significant covariate.
Conclusion: The population PK of TLD-1, including its release and main metabolite, were successfully characterised using non-compartmental and compartmental analyses. Based on its long half-life, TLD-1 presents a promising candidate for further clinical development. The PK characteristics form the basis to investigate TLD-1 exposure-response (i.e., clinical efficacy) and exposure-toxicity relationships in the future. Once such relationships have been established, the developed population PK model can be further used in model-informed precision dosing strategies.
Clinical trial registration: ClinicalTrials.gov-NCT03387917-January 2, 2018.
Keywords: Doxorubicin; Liposomes; Nanoparticles; Nonlinear mixed-effects model; Pharmacokinetics; Pharmacometrics.
© 2024. The Author(s).
Conflict of interest statement
C.K. and W.H. report grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, Astra Zeneca Ltd., Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA, Novo Nordisk and Sanofi) for the PharMetrX PhD program. C.K. reports grants for the Innovative Medicines Initiative-Joint Undertaking (“DDMoRe”), Diurnal Ltd., the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (JPIAMR) and from the European Commission within the Horizon 2020 framework program (“FAIR”). A.M.L. is a current employee of Pharmetheus AB and a paid consultant to multiple pharmaceutical companies. I.C. provided advisory/expert opinion for GSK, Novartis, Astra Zeneca, and MSD and recceived travel grants from Tesaro and institutional grants for clinical trials (Principal Investigator): MSD, Bayer, Oasmia. A.T. received institutional research funding from Innomedica, MEI Pharma, Merck, Bayer, Roche, Novartis, Pfizer, ADC Therapeutics, and Eli Lilly, and consulting fees from Bayer, Eli Lilly, Roche, and Novartis. M.J. is investigators in clinical trials for AstraZeneca, Basilea Pharmaceutica, Bayer, BMS, Daiichi Sankyo, Immunophotonics, Innomedia, Janssen, Lilly, MSD, Novartis, Pfizer, Pharmamar, Roche, Sanofi, Takeda, and received travel grants from BSM, Roche, MSD. S.H. provided adivsory/expert opinion for Bayer, Novartis, Lilly, AstraZeneca, and MSD. A.S. received institutional funding for clinical trials for AbbVie, ADC Therapeutics, Amgen, AstraZeneca, Bayer, Cellestia, Incyte, Loxo Oncology, Merck MSD, Novartis, Pfizer, Philogen and Roche, provided paid consultancy services for Debiopharm, Janssen, AstraZeneca, Incyte, Eli Lilly, Novartis, Roche, and Lox Oncology, and received travel grants from Incyte and AstraZeneca. The other authors declare that they have no conflict of interest.
Figures

Similar articles
-
Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years.Cancer Chemother Pharmacol. 2013 Mar;71(3):749-63. doi: 10.1007/s00280-013-2069-1. Epub 2013 Jan 13. Cancer Chemother Pharmacol. 2013. PMID: 23314734
-
Plasmafiltration as a possible contributor to kinetic targeting of pegylated liposomal doxorubicin (PLD) in order to prevent organ toxicity and immunosuppression.Cancer Chemother Pharmacol. 2016 Feb;77(2):429-37. doi: 10.1007/s00280-015-2936-z. Epub 2015 Dec 17. Cancer Chemother Pharmacol. 2016. PMID: 26678853
-
Pharmacokinetics and its relation to toxicity of pegylated-liposomal doxorubicin in Chinese patients with breast tumours.J Clin Pharm Ther. 2010 Oct;35(5):593-601. doi: 10.1111/j.1365-2710.2009.01128.x. J Clin Pharm Ther. 2010. PMID: 20831683 Clinical Trial.
-
Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours.Drugs. 1997;54 Suppl 4:15-21. doi: 10.2165/00003495-199700544-00005. Drugs. 1997. PMID: 9361957 Review.
-
Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies.Clin Pharmacokinet. 2003;42(5):419-36. doi: 10.2165/00003088-200342050-00002. Clin Pharmacokinet. 2003. PMID: 12739982 Review.
Cited by
-
Nanoparticle technologies in precision oncology and personalized vaccine development: Challenges and advances.Int J Pharm X. 2025 Jul 5;10:100353. doi: 10.1016/j.ijpx.2025.100353. eCollection 2025 Dec. Int J Pharm X. 2025. PMID: 40688030 Free PMC article. Review.
References
-
- Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65:157–170. 10.1111/j.2042-7158.2012.01567.x - PubMed
-
- Speth PAJ, van Hoesel QGCM, Haanen C (1988) Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet 14:287–310. 10.2165/00003088-198814050-00002 - PubMed
-
- Joerger M, Huitema ADR, Meenhorst PL et al (2005) Pharmacokinetics of low-dose doxorubicin and metabolites in patients with AIDS-related Kaposi sarcoma. Cancer Chemother Pharmacol 55:488–496. 10.1007/s00280-004-0900-4 - PubMed
-
- O’Brien MER, Wigler N, Inbar M et al (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYXTM/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15:440–449. 10.1093/annonc/mdh097 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

