Practices and Barriers in Developing and Disseminating Plain-Language Resources Reporting Medical Research Information: A Scoping Review
- PMID: 38878237
- PMCID: PMC11343906
- DOI: 10.1007/s40271-024-00700-y
Practices and Barriers in Developing and Disseminating Plain-Language Resources Reporting Medical Research Information: A Scoping Review
Abstract
Background: The intent of plain-language resources (PLRs) reporting medical research information is to advance health literacy among the general public and enable them to participate in shared decision-making (SDM). Regulatory mandates coupled with academic and industry initiatives have given rise to an increasing volume of PLRs summarizing medical research information. However, there is significant variability in the quality, format, readability, and dissemination channels for PLRs. In this scoping review, we identify current practices, guidance, and barriers in developing and disseminating PLRs reporting medical research information to the general public including patients and caregivers. We also report on the PLR preferences of these intended audiences.
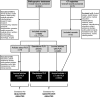
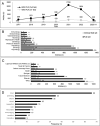
Methods: A literature search of three bibliographic databases (PubMed, EMBASE, Web of Science) and three clinical trial registries (NIH, EMA, ISRCTN registry) was performed. Snowball searches within reference lists of primary articles were added. Articles with PLRs or reporting topics related to PLRs use and development available between January 2017 and June 2023 were identified. Evidence mapping and synthesis were used to make qualitative observations. Identified PLRs were quantitatively assessed, including temporal annual trends, availability by field of medicine, language, and publisher types.
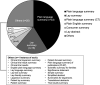
Results: A total of 9116 PLRs were identified, 9041 from the databases and 75 from clinical trial registries. The final analysis included 6590 PLRs from databases and 72 from registries. Reported barriers to PLR development included ambiguity in guidance, lack of incentives, and concerns of researchers writing for the general public. Available guidance recommendations called for greater dissemination, increased readability, and varied content formats. Patients preferred visual PLRs formats (e.g., videos, comics), which were easy to access on the internet and used short jargon-free text. In some instances, older audiences and more educated readers preferred text-only PLRs. Preferences among the general public were mostly similar to those of patients. Psychology, followed by oncology, showed the highest number of PLRs, predominantly from academia-sponsored research. Text-only PLRs were most commonly available, while graphical, digital, or online formats were less available. Preferred dissemination channels included paywall-free journal websites, indexing on PubMed, third-party websites, via email to research participants, and social media.
Conclusions: This scoping review maps current practices, recommendations, and patients' and the general public's preferences for PLR development and dissemination. The results suggest that making PLRs available to a wider audience by improving nomenclature, accessibility, and providing translations may contribute to empowerment and SDM. Minimizing variability among available guidance for PLR development may play an important role in amplifying the value and impact of these resources.
Plain language summary
Plain-language resources (PLRs) can help people understand medical research information. This will allow them to make informed decisions about their health. However, PLRs vary in quality, format, and ways in which they are shared. In this study, researchers looked at how PLRs are made and publicly shared. They also studied what makes PLRs useful for the public and patients. Creating PLRs is not easy because of unclear guidelines on writing for the public. Using different formats and languages can make PLRs readable. Patients preferred PLRs as videos and comics. Older and educated readers liked text-only PLRs. The fields of psychology and oncology had the highest number of PLRs. Text-only PLRs were more common than digital or online formats. PLRs should be easily and freely accessible. Open-access journal websites, PubMed, third-party websites, email, and social media can be used to share PLRs. This study showed that PLRs can be helpful, but there are challenges in creating and sharing them. Good PLRs can inform patients and help them make better health-related decisions.
© 2024. The Author(s).
Conflict of interest statement
AP is a full-time employee of Novartis Pharma AG. However, this work is independent of his employment and is part of his doctoral research at the Institute for Biomedical Ethics, University of Basel. IA, TW, and BE have no competing interests to disclose.
Figures
Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Open Pharma recommendations for plain language summaries of peer-reviewed medical journal publications.Curr Med Res Opin. 2021 Nov;37(11):2015-2016. doi: 10.1080/03007995.2021.1971185. Epub 2021 Sep 12. Curr Med Res Opin. 2021. Update in: Curr Med Res Opin. 2022 Jun;38(6):881-882. doi: 10.1080/03007995.2022.2072570. PMID: 34511020 Updated.
-
Optimizing Readability and Format of Plain Language Summaries for Medical Research Articles: Cross-sectional Survey Study.J Med Internet Res. 2022 Jan 11;24(1):e22122. doi: 10.2196/22122. J Med Internet Res. 2022. PMID: 35014966 Free PMC article.
-
What Author Instructions Do Health Journals Provide for Writing Plain Language Summaries? A Scoping Review.Patient. 2023 Jan;16(1):31-42. doi: 10.1007/s40271-022-00606-7. Epub 2022 Oct 27. Patient. 2023. PMID: 36301440 Free PMC article.
-
The power of language: how to bridge the gap between healthcare research and patients - a scoping review.Curr Med Res Opin. 2024 Feb;40(2):279-291. doi: 10.1080/03007995.2023.2295984. Epub 2024 Jan 24. Curr Med Res Opin. 2024. PMID: 38131338
Cited by
-
The Dementia Literacy Assessment (DeLA): A novel measure of Alzheimer's disease and related disorders health literacy in diverse populations.Alzheimers Dement (N Y). 2025 Feb 19;11(1):e70054. doi: 10.1002/trc2.70054. eCollection 2025 Jan-Mar. Alzheimers Dement (N Y). 2025. PMID: 39975469 Free PMC article.
-
Assessing the Capability of Large Language Model Chatbots in Generating Plain Language Summaries.Cureus. 2025 Mar 21;17(3):e80976. doi: 10.7759/cureus.80976. eCollection 2025 Mar. Cureus. 2025. PMID: 40260353 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

