Weighted Breaths: Exploring Biologic and Non-Biologic Therapies for Co-Existing Asthma and Obesity
- PMID: 38878250
- PMCID: PMC11233394
- DOI: 10.1007/s11882-024-01153-x
Weighted Breaths: Exploring Biologic and Non-Biologic Therapies for Co-Existing Asthma and Obesity
Abstract
Purpose of review: To discuss the effectiveness of biologics, some of which comprise the newest class of asthma controller medications, and non-biologics in the treatment of asthma co-existing with obesity.
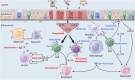
Recent findings: Our review of recent preliminary and published data from clinical trials revealed that obese asthmatics respond favorably to dupilumab, mepolizumab, omalizumab, and tezepelumab, which are biologics currently indicated as add-on maintenance therapy for severe asthma. Furthermore, clinical trials are ongoing to assess the efficacy of non-biologics in the treatment of obese asthma, including a glucagon-like peptide-1 receptor agonist, a Janus kinase inhibitor, and probiotics. Although many biologics presently indicated as add-on maintenance therapy for severe asthma exhibit efficacy in obese asthmatics, other phenotypes of asthma co-existing with obesity may be refractory to these medications. Thus, to improve quality of life and asthma control, it is imperative to identify therapeutic options for all existing phenotypes of obese asthma.
Keywords: Asthma; Biologic; Clinical trial; Forced expiratory volume in one second; Obesity; Probiotic.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Albert W. Pilkington, IV and Richard A. Johnston are employees of the federal government of the United States of America. Bhanusowmya Buragamadagu declares no conflict of interest.
Figures
Similar articles
-
Efficacy of Biologics in Patients with Allergic Severe Asthma, Overall and by Blood Eosinophil Count: A Literature Review.Adv Ther. 2023 Nov;40(11):4721-4740. doi: 10.1007/s12325-023-02647-2. Epub 2023 Sep 12. Adv Ther. 2023. PMID: 37698716 Free PMC article. Review.
-
The phenotypic heterogeneity of obese and nonobese patients with severe asthma and comparison of omalizumab-mepolizumab treatment efficiency in these patients.Medicine (Baltimore). 2023 Oct 27;102(43):e35247. doi: 10.1097/MD.0000000000035247. Medicine (Baltimore). 2023. PMID: 37904405 Free PMC article.
-
Pairwise indirect treatment comparison of dupilumab versus other biologics in patients with uncontrolled persistent asthma.Respir Med. 2022 Jan;191:105991. doi: 10.1016/j.rmed.2020.105991. Epub 2020 Apr 29. Respir Med. 2022. PMID: 35090688
-
Real-World Studies of Biologics for the Treatment of Moderate-to-Severe Asthma.Immunol Allergy Clin North Am. 2024 Nov;44(4):737-750. doi: 10.1016/j.iac.2024.07.007. Immunol Allergy Clin North Am. 2024. PMID: 39389721 Review.
-
The new biologic drugs: Which children with asthma should get what?Pediatr Pulmonol. 2024 Dec;59(12):3057-3074. doi: 10.1002/ppul.27218. Epub 2024 Sep 13. Pediatr Pulmonol. 2024. PMID: 39267467 Free PMC article. Review.
Cited by
-
The clinical and pathological histology efficacy of biological therapy for severe asthma with a phenotype of type 2 inflammation - systematic review.Front Immunol. 2025 Apr 15;16:1531986. doi: 10.3389/fimmu.2025.1531986. eCollection 2025. Front Immunol. 2025. PMID: 40303400 Free PMC article.
-
Obesity: Next game changer of allergic airway diseases?Clin Transl Med. 2025 May;15(5):e70316. doi: 10.1002/ctm2.70316. Clin Transl Med. 2025. PMID: 40329860 Free PMC article. Review.
References
-
- Panuganti KK, Nguyen M, Kshirsagar RK. Obesity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK459357/. - PubMed
-
- World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight Accessed April 4 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

