NLRC5 senses NAD+ depletion, forming a PANoptosome and driving PANoptosis and inflammation
- PMID: 38878777
- PMCID: PMC11283362
- DOI: 10.1016/j.cell.2024.05.034
NLRC5 senses NAD+ depletion, forming a PANoptosome and driving PANoptosis and inflammation
Abstract
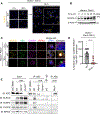
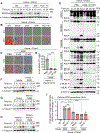
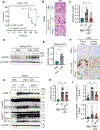
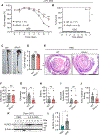
NLRs constitute a large, highly conserved family of cytosolic pattern recognition receptors that are central to health and disease, making them key therapeutic targets. NLRC5 is an enigmatic NLR with mutations associated with inflammatory and infectious diseases, but little is known about its function as an innate immune sensor and cell death regulator. Therefore, we screened for NLRC5's role in response to infections, PAMPs, DAMPs, and cytokines. We identified that NLRC5 acts as an innate immune sensor to drive inflammatory cell death, PANoptosis, in response to specific ligands, including PAMP/heme and heme/cytokine combinations. NLRC5 interacted with NLRP12 and PANoptosome components to form a cell death complex, suggesting an NLR network forms similar to those in plants. Mechanistically, TLR signaling and NAD+ levels regulated NLRC5 expression and ROS production to control cell death. Furthermore, NLRC5-deficient mice were protected in hemolytic and inflammatory models, suggesting that NLRC5 could be a potential therapeutic target.
Keywords: ASC; DAMP; NLRC5; NLRP12; NLRP3; PAMP; PANoptosis; RIPK3; ROS; TLRs; TNF; apoptosis; caspase; colitis; heme; hemophagocytic lymphohistiocytosis; inflammasome; inflammatory cell death; necroptosis; pyroptosis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests St. Jude Children’s Research Hospital filed a provisional patent application on methods for modulating NLRP12 and NLRC5 described in this study, listing B.S. and T.-D.K. as inventors (serial no. 63/501,430). The PCT application was published with the World Intellectual Property Organization (WO 2024/097571 A1).
Figures


References
-
- Harton JA, Linhoff MW, Zhang J, and Ting JP (2002). Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol 169, 4088–4093. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

