Mutation-induced shift of the photosystem II active site reveals insight into conserved water channels
- PMID: 38879008
- PMCID: PMC11294709
- DOI: 10.1016/j.jbc.2024.107475
Mutation-induced shift of the photosystem II active site reveals insight into conserved water channels
Abstract
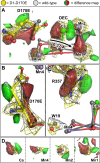
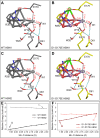
Photosystem II (PSII) is the water-plastoquinone photo-oxidoreductase central to oxygenic photosynthesis. PSII has been extensively studied for its ability to catalyze light-driven water oxidation at a Mn4CaO5 cluster called the oxygen-evolving complex (OEC). Despite these efforts, the complete reaction mechanism for water oxidation by PSII is still heavily debated. Previous mutagenesis studies have investigated the roles of conserved amino acids, but these studies have lacked a direct structural basis that would allow for a more meaningful interpretation. Here, we report a 2.14-Å resolution cryo-EM structure of a PSII complex containing the substitution Asp170Glu on the D1 subunit. This mutation directly perturbs a bridging carboxylate ligand of the OEC, which alters the spectroscopic properties of the OEC without fully abolishing water oxidation. The structure reveals that the mutation shifts the position of the OEC within the active site without markedly distorting the Mn4CaO5 cluster metal-metal geometry, instead shifting the OEC as a rigid body. This shift disturbs the hydrogen-bonding network of structured waters near the OEC, causing disorder in the conserved water channels. This mutation-induced disorder appears consistent with previous FTIR spectroscopic data. We further show using quantum mechanics/molecular mechanics methods that the mutation-induced structural changes can affect the magnetic properties of the OEC by altering the axes of the Jahn-Teller distortion of the Mn(III) ion coordinated to D1-170. These results offer new perspectives on the conserved water channels, the rigid body property of the OEC, and the role of D1-Asp170 in the enzymatic water oxidation mechanism.
Keywords: cryo-EM; hydrogen-bond network; mutagenesis; oxygen-evolving complex; photosynthesis; photosystem II; quantum mechanics/molecular mechanics; water channel.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Perturbing the water cavity surrounding the manganese cluster by mutating the residue D1-valine 185 has a strong effect on the water oxidation mechanism of photosystem II.Biochemistry. 2013 Oct 1;52(39):6824-33. doi: 10.1021/bi400930g. Epub 2013 Sep 16. Biochemistry. 2013. PMID: 24010490
-
Photosystem II oxygen-evolving complex photoassembly displays an inverse H/D solvent isotope effect under chloride-limiting conditions.Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18917-18922. doi: 10.1073/pnas.1910231116. Epub 2019 Sep 4. Proc Natl Acad Sci U S A. 2019. PMID: 31484762 Free PMC article.
-
Substitution of the D1-Asn87 site in photosystem II of cyanobacteria mimics the chloride-binding characteristics of spinach photosystem II.J Biol Chem. 2018 Feb 16;293(7):2487-2497. doi: 10.1074/jbc.M117.813170. Epub 2017 Dec 20. J Biol Chem. 2018. PMID: 29263091 Free PMC article.
-
S1-state Mn4Ca complex of Photosystem II exists in equilibrium between the two most-stable isomeric substates: XRD and EXAFS evidence.J Photochem Photobiol B. 2011 Jul-Aug;104(1-2):100-10. doi: 10.1016/j.jphotobiol.2011.03.002. Epub 2011 Mar 13. J Photochem Photobiol B. 2011. PMID: 21592813 Review.
Cited by
-
Structure of a mutated photosystem II complex reveals changes to the hydrogen-bonding network that affect proton egress during O-O bond formation.J Biol Chem. 2025 Mar;301(3):108272. doi: 10.1016/j.jbc.2025.108272. Epub 2025 Feb 6. J Biol Chem. 2025. PMID: 39922494 Free PMC article.
-
Electron Transfer Mechanism from the Oxygen-Evolving Complex to the Electron-Acceptor Tyrosine during the S2 to S3 Transition in Photosystem II.ACS Omega. 2025 Jun 3;10(23):24461-24471. doi: 10.1021/acsomega.5c00945. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547619 Free PMC article.
References
-
- Blankenship R.E. Molecular Mechanisms of Photosynthesis. 3rd ed. John Wiley & Sons; Hoboken, NJ: 2021.
-
- Cox N., Pantazis D.A., Lubitz W. Current understanding of the mechanism of water oxidation in photosystem II and its relation to XFEL data. Annu. Rev. Biochem. 2020;89:795–820. - PubMed
-
- Vinyard D.J., Brudvig G.W. Progress toward a molecular mechanism of water oxidation in photosystem II. Annu. Rev. Phys. Chem. 2017;68:101–116. - PubMed

