Inhibition of tartrate-resistant acid phosphatase 5 can prevent cardiac fibrosis after myocardial infarction
- PMID: 38879488
- PMCID: PMC11179352
- DOI: 10.1186/s10020-024-00856-1
Inhibition of tartrate-resistant acid phosphatase 5 can prevent cardiac fibrosis after myocardial infarction
Abstract
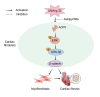
Background: Myocardial infarction (MI) leads to enhanced activity of cardiac fibroblasts (CFs) and abnormal deposition of extracellular matrix proteins, resulting in cardiac fibrosis. Tartrate-resistant acid phosphatase 5 (ACP5) has been shown to promote cell proliferation and phenotypic transition. However, it remains unclear whether ACP5 is involved in the development of cardiac fibrosis after MI. The present study aimed to investigate the role of ACP5 in post-MI fibrosis and its potential underlying mechanisms.
Methods: Clinical blood samples were collected to detect ACP5 concentration. Myocardial fibrosis was induced by ligation of the left anterior descending coronary artery. The ACP5 inhibitor, AubipyOMe, was administered by intraperitoneal injection. Cardiac function and morphological changes were observed on Day 28 after injury. Cardiac CFs from neonatal mice were extracted to elucidate the underlying mechanism in vitro. The expression of ACP5 was silenced by small interfering RNA (siRNA) and overexpressed by adeno-associated viruses to evaluate its effect on CF activation.
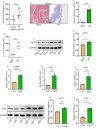
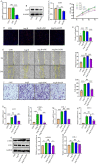
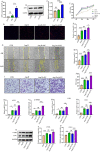
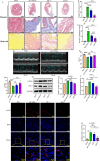
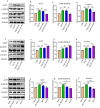
Results: The expression of ACP5 was increased in patients with MI, mice with MI, and mice with Ang II-induced fibrosis in vitro. AubipyOMe inhibited cardiac fibrosis and improved cardiac function in mice after MI. ACP5 inhibition reduced cell proliferation, migration, and phenotypic changes in CFs in vitro, while adenovirus-mediated ACP5 overexpression had the opposite effect. Mechanistically, the classical profibrotic pathway of glycogen synthase kinase-3β (GSK3β)/β-catenin was changed with ACP5 modulation, which indicated that ACP5 had a positive regulatory effect. Furthermore, the inhibitory effect of ACP5 deficiency on the GSK3β/β-catenin pathway was counteracted by an ERK activator, which indicated that ACP5 regulated GSK3β activity through ERK-mediated phosphorylation, thereby affecting β-catenin degradation.
Conclusion: ACP5 may influence the proliferation, migration, and phenotypic transition of CFs, leading to the development of myocardial fibrosis after MI through modulating the ERK/GSK3β/β-catenin signaling pathway.
Keywords: Cardiac fibroblasts (CFs); Cardiac fibrosis; Glycogen synthase kinase-3β (GSK3β)/β-catenin signaling pathway; Myocardial infarction; Tartrate-resistant acid phosphatase 5 (ACP5).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Castillo-Casas JM, Caño-Carrillo S, Sánchez-Fernández C, Franco D, Lozano-Velasco E. Comparative analysis of Heart Regeneration: searching for the Key to heal the heart-part II: Molecular mechanisms of Cardiac Regeneration. J Cardiovasc Dev Dis. 2023;10(9):357. doi: 10.3390/jcdd10090357. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- No. 81070125, 81270213, 81670306/the National Natural Science Foundation of China
- No. 2010B031600032, 2014A020211002/the Science and Technology Foundation in Guangdong Province
- No. 2017A030313503/the National Natural Science Foundation of Guangdong Province
- No. 201806020084/the Science and Technology Foundation in Guangzhou City
- No. 13ykzd16, 17ykjc18/Fundamental Research Funds for the Central Universities of Beijing University of Chemical Technology
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

