Safety and efficacy analysis of neoadjuvant pertuzumab, trastuzumab and standard chemotherapy for HER2-positive early breast cancer: real-world data from NeoPowER study
- PMID: 38879498
- PMCID: PMC11179289
- DOI: 10.1186/s12885-024-12506-0
Safety and efficacy analysis of neoadjuvant pertuzumab, trastuzumab and standard chemotherapy for HER2-positive early breast cancer: real-world data from NeoPowER study
Abstract
Background: The addition of pertuzumab (P) to trastuzumab (H) and standard chemotherapy (CT) as neoadjuvant treatment (NaT) for patients with HER2 + breast cancer (BC), has shown to increase the pathological complete response (pCR) rate, without main safety concerns. The aim of NeoPowER trial is to evaluate safety and efficacy of P + H + CT in a real-world population.
Methods: We retrospectively reviewed the medical records of stage II-III, HER2 + BC patients treated with NaT: who received P + H + CT (neopower group) in 5 Emilia Romagna institutions were compared with an historical group who received H + CT (control group). The primary endpoint was the safety, secondary endpoints were pCR rate, DRFS and OS and their correlation to NaT and other potential variables.
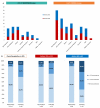
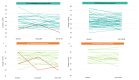
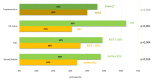
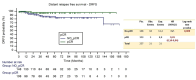
Results: 260 patients were included, 48% received P + H + CT, of whom 44% was given anthraciclynes as part of CT, compared to 83% in the control group. The toxicity profile was similar, excluding diarrhea more frequent in the neopower group (20% vs. 9%). Three patients experienced significant reductions in left ventricular ejection fraction (LVEF), all receiving anthracyclines. The pCR rate was 46% (P + H + CT) and 40% (H + CT) (p = 0.39). The addition of P had statistically correlation with pCR only in the patients receiving anthra-free regimens (OR = 3.05,p = 0.047). Preoperative use of anthracyclines (OR = 1.81,p = 0.03) and duration of NaT (OR = 1.18,p = 0.02) were statistically related to pCR. 12/21 distant-relapse events and 14/17 deaths occurred in the control group. Patients who achieve pCR had a significant increase in DRFS (HR = 0.23,p = 0.009).
Conclusions: Adding neoadjuvant P to H and CT is safe. With the exception of diarrhea, rate of adverse events of grade > 2 did not differ between the two groups. P did not increase the cardiotoxicity when added to H + CT, nevertheless in our population all cardiac events occurred in patients who received anthracycline-containing regimens. Not statistically significant, higher pCR rate is achievable in patients receiving neoadjuvant P + H + CT. The study did not show a statistically significant correlation between the addition of P and long-term outcomes.
Keywords: Early breast cancer; HER2 dual blockade; HER2+; Neoadjuvant treatment; Pertuzumab; Real world data.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial.
-
Real-world experience with pertuzumab and trastuzumab combined with chemotherapy in neoadjuvant treatment for patients with early-stage HER2-positive breast cancer: the NEOPERSUR study.Clin Transl Oncol. 2024 Sep;26(9):2217-2226. doi: 10.1007/s12094-024-03440-5. Epub 2024 Mar 28. Clin Transl Oncol. 2024. PMID: 38538968 Free PMC article.
-
Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2018 Dec;19(12):1630-1640. doi: 10.1016/S1470-2045(18)30570-9. Epub 2018 Nov 6. Lancet Oncol. 2018. PMID: 30413379 Clinical Trial.
-
Efficacy and safety of HER2 inhibitors in combination with or without pertuzumab for HER2-positive breast cancer: a systematic review and meta-analysis.BMC Cancer. 2019 Oct 21;19(1):973. doi: 10.1186/s12885-019-6132-0. BMC Cancer. 2019. PMID: 31638935 Free PMC article.
-
Dual HER2 Blockade in Neoadjuvant Treatment of HER2+ Breast Cancer: A Meta-Analysis and Review.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820960721. doi: 10.1177/1533033820960721. Technol Cancer Res Treat. 2020. PMID: 32990165 Free PMC article. Review.
Cited by
-
The Benefits and Safety of Monoclonal Antibodies: Implications for Cancer Immunotherapy.J Inflamm Res. 2025 Mar 24;18:4335-4357. doi: 10.2147/JIR.S499403. eCollection 2025. J Inflamm Res. 2025. PMID: 40162076 Free PMC article. Review.
-
A phase I, randomized, double-blind, parallel, single-dose pharmacokinetic study to evaluate the biosimilarity of KM118 (proposed pertuzumab biosimilar) with reference pertuzumab (Perjeta®) in healthy male subjects.Ann Med. 2025 Dec;57(1):2523561. doi: 10.1080/07853890.2025.2523561. Epub 2025 Jun 28. Ann Med. 2025. PMID: 40580300 Free PMC article. Clinical Trial.
References
-
- Linee Guida AIOM. Carcinoma Mammario in Stadio Precoce. 2023.
-
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-Positive locally advanced breast cancer (the NOAH Trial): a randomised controlled superiority trial with a parallel HER2-Negative cohort. 2010;375:8. - PubMed
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast Cancer: the CTNeoBC Pooled Analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Matthews CM, Nymberg K, Berger M, Vargo CA, Dempsey J, Li J, Ramaswamy B, Reinbolt R, Sardesai S, Wesolowski R, et al. Pathological complete response rates with Pertuzumab-based neoadjuvant chemotherapy in breast Cancer: a single-center experience. J Oncol Pharm Pract. 2020;26:572–9. doi: 10.1177/1078155219857800. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

