Cystatin F attenuates neuroinflammation and demyelination following murine coronavirus infection of the central nervous system
- PMID: 38879499
- PMCID: PMC11179388
- DOI: 10.1186/s12974-024-03153-0
Cystatin F attenuates neuroinflammation and demyelination following murine coronavirus infection of the central nervous system
Abstract
Background: Cystatin F is a secreted lysosomal cysteine protease inhibitor that has been implicated in affecting the severity of demyelination and enhancing remyelination in pre-clinical models of immune-mediated demyelination. How cystatin F impacts neurologic disease severity following viral infection of the central nervous system (CNS) has not been well characterized and was the focus of this study. We used cystatin F null-mutant mice (Cst7-/-) with a well-established model of murine coronavirus-induced neurologic disease to evaluate the contributions of cystatin F in host defense, demyelination and remyelination.
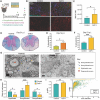
Methods: Wildtype controls and Cst7-/- mice were intracranially (i.c.) infected with a sublethal dose of the neurotropic JHM strain of mouse hepatitis virus (JHMV), with disease progression and survival monitored daily. Viral plaque assays and qPCR were used to assess viral levels in CNS. Immune cell infiltration into the CNS and immune cell activation were determined by flow cytometry and 10X genomics chromium 3' single cell RNA sequencing (scRNA-seq). Spinal cord demyelination was determined by luxol fast blue (LFB) and Hematoxylin/Eosin (H&E) staining and axonal damage assessed by immunohistochemical staining for SMI-32. Remyelination was evaluated by electron microscopy (EM) and calculation of g-ratios.
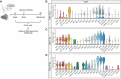
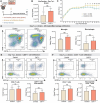
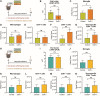
Results: JHMV-infected Cst7-/- mice were able to control viral replication within the CNS, indicating that cystatin F is not essential for an effective Th1 anti-viral immune response. Infiltration of T cells into the spinal cords of JHMV-infected Cst7-/- mice was increased compared to infected controls, and this correlated with increased axonal damage and demyelination associated with impaired remyelination. Single-cell RNA-seq of CD45 + cells enriched from spinal cords of infected Cst7-/- and control mice revealed enhanced expression of transcripts encoding T cell chemoattractants, Cxcl9 and Cxcl10, combined with elevated expression of interferon-g (Ifng) and perforin (Prf1) transcripts in CD8 + T cells from Cst7-/- mice compared to controls.
Conclusions: Cystatin F is not required for immune-mediated control of JHMV replication within the CNS. However, JHMV-infected Cst7-/- mice exhibited more severe clinical disease associated with increased demyelination and impaired remyelination. The increase in disease severity was associated with elevated expression of T cell chemoattractant chemokines, concurrent with increased neuroinflammation. These findings support the idea that cystatin F influences expression of proinflammatory gene expression impacting neuroinflammation, T cell activation and/or glia cell responses ultimately impacting neuroinflammation and neurologic disease.
Keywords: Coronavirus; Cystatin F; Demyelination; Microglia; Remyelination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system.Glia. 2020 Nov;68(11):2345-2360. doi: 10.1002/glia.23844. Epub 2020 May 25. Glia. 2020. PMID: 32449994 Free PMC article.
-
FTY720 (fingolimod) modulates the severity of viral-induced encephalomyelitis and demyelination.J Neuroinflammation. 2014 Aug 20;11:138. doi: 10.1186/s12974-014-0138-y. J Neuroinflammation. 2014. PMID: 25138356 Free PMC article.
-
Ablation of microglia following infection of the central nervous system with a neurotropic murine coronavirus infection leads to increased demyelination and impaired remyelination.J Neuroimmunol. 2023 Aug 15;381:578133. doi: 10.1016/j.jneuroim.2023.578133. Epub 2023 Jun 17. J Neuroimmunol. 2023. PMID: 37352687 Free PMC article.
-
Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease.Viral Immunol. 2019 Jan/Feb;32(1):25-37. doi: 10.1089/vim.2018.0073. Epub 2018 Aug 15. Viral Immunol. 2019. PMID: 30109979 Free PMC article. Review.
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
Cited by
-
Embryonic macrophages orchestrate niche cell homeostasis for the establishment of the definitive hematopoietic stem cell pool.Nat Commun. 2025 May 14;16(1):4428. doi: 10.1038/s41467-025-59059-9. Nat Commun. 2025. PMID: 40368907 Free PMC article.
-
Cystatin F-a key player in central nervous system disease.J Neuroinflammation. 2025 Aug 14;22(1):203. doi: 10.1186/s12974-025-03526-z. J Neuroinflammation. 2025. PMID: 40813983 Review.
References
-
- Glass WG, Hickey MJ, Hardison JL, Liu MT, Manning JE, Lane TE. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–25. doi: 10.4049/jimmunol.172.7.4018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

