Fetal hemoglobin induction in azacytidine responders enlightens methylation patterns related to blast clearance in higher-risk MDS and CMML
- PMID: 38879530
- PMCID: PMC11180405
- DOI: 10.1186/s13148-024-01687-x
Fetal hemoglobin induction in azacytidine responders enlightens methylation patterns related to blast clearance in higher-risk MDS and CMML
Abstract
Background: As new treatment options for patients with higher-risk myelodysplastic syndromes are emerging, identification of prognostic markers for hypomethylating agent (HMA) treatment and understanding mechanisms of their delayed and short-term responses are essential. Early fetal hemoglobin (HbF) induction has been suggested as a prognostic indicator for decitabine-treated patients. Although epigenetic mechanisms are assumed, responding patients' epigenomes have not been thoroughly examined. We aimed to clarify HbF kinetics and prognostic value for azacytidine treated patients, as well as the epigenetic landscape that might influence HbF re-expression and its clinical relevance.
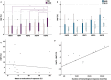
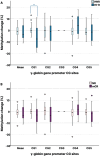
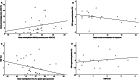
Results: Serial HbF measurements by high-performance liquid chromatography (n = 20) showed induction of HbF only among responders (p = 0.030). Moreover, HbF increase immediately after the first azacytidine cycle demonstrated prognostic value for progression-free survival (PFS) (p = 0.032, HR = 0.19, CI 0.24-1.63). Changes in methylation patterns were revealed with methylated DNA genome-wide sequencing analysis (n = 7) for FOG-1, RCOR-1, ZBTB7A and genes of the NuRD-complex components. Targeted pyrosequencing methodology (n = 28) revealed a strong inverse correlation between the degree of γ-globin gene (HBG2) promoter methylation and baseline HbF levels (p = 0.003, rs = - 0.663). A potential epigenetic mechanism of HbF re-expression in azacytidine responders was enlightened by targeted methylation analysis, through hypomethylation of site -53 of HBG2 promoter (p = 0.039, rs = - 0.504), which corresponds to MBD2-NuRD binding site, and to hypermethylation of the CpG326 island of ZBTB7A (p = 0.05, rs = 0.482), a known HbF repressor. These changes were associated to blast cell clearance (pHBG2 = 0.011, rs = 0.480/pZBTB7A = 0.026, rs = 0.427) and showed prognostic value for PFS (pZBTB7A = 0.037, HR = 1.14, CI 0.34-3.8).
Conclusions: Early HbF induction is featured as an accessible prognostic indicator for HMA treatment and the proposed potential epigenetic mechanism of HbF re-expression in azacytidine responders includes hypomethylation of the γ-globin gene promoter region and hypermethylation of the CpG326 island of ZBTB7A. The association of these methylation patterns with blast clearance and their prognostic value for PFS paves the way to discuss in-depth azacytidine epigenetic mechanism of action.
Keywords: Fetal hemoglobin; Methylation patterns; Myelodysplastic syndromes; Prognosis; ZBTB7A.
© 2024. The Author(s).
Conflict of interest statement
A.Sy. has conducted clinical trials and has received research funding through the University of Patras, honoraria and/or travel expenses by Abbvie, Agios, Amgen, Astra-Zeneca, BMS, Incyte/Genesis, Gilead, GSK, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, Sobi and Takeda. A.K. has conducted clinical trials and has received honoraria and/or travel expenses by Abbvie, Agios, Demo/ApoPharma, BMS, Incyte/Genesis, Janssen, Novartis, Pfizer and Sobi. VL has received honoraria and/or travel expenses by Abbvie, Amgen, Demo/ApoPharma, GSK, Incyte/Genesis, Janssen, Sanofi and Win-Medica. EV has received honoraria and/or travel expenses by Abbvie, Gilead and Pfizer. R.B. and J.G. are shareholders in Methylomics B.V., a commercial company that applies MeD-seq to develop methylation markers for cancer staging. The remaining co-authors have nothing to disclose.
Figures



Similar articles
-
Elevated fetal haemoglobin is a predictor of better outcome in MDS/AML patients receiving 5-aza-2'-deoxycytidine (Decitabine).Br J Haematol. 2017 Feb;176(4):609-617. doi: 10.1111/bjh.14463. Epub 2016 Dec 1. Br J Haematol. 2017. PMID: 27905102 Clinical Trial.
-
RNA methylation sequencing shows different gene expression signatures for response to azacytidine therapy in high-grade myelodysplastic syndromes.J Cell Mol Med. 2024 Sep;28(18):e70078. doi: 10.1111/jcmm.70078. J Cell Mol Med. 2024. PMID: 39334509 Free PMC article.
-
In vivo effects of decitabine in myelodysplasia and acute myeloid leukemia: review of cytogenetic and molecular studies.Ann Hematol. 2005 Dec;84 Suppl 1:32-8. doi: 10.1007/s00277-005-0004-1. Ann Hematol. 2005. PMID: 16292549 Review.
-
Impact of ZBTB7A hypomethylation and expression patterns on treatment response to hydroxyurea.Hum Genomics. 2018 Oct 1;12(1):45. doi: 10.1186/s40246-018-0177-z. Hum Genomics. 2018. PMID: 30285874 Free PMC article.
-
Digging deep into "dirty" drugs - modulation of the methylation machinery.Drug Metab Rev. 2015 May;47(2):252-79. doi: 10.3109/03602532.2014.995379. Epub 2015 Jan 8. Drug Metab Rev. 2015. PMID: 25566693 Free PMC article. Review.
Cited by
-
Integrating advanced analytical methods to assess epigenetic marks affecting response to hypomethylating agents in higher risk myelodysplastic syndrome.Mol Med. 2025 Feb 14;31(1):59. doi: 10.1186/s10020-025-01123-7. Mol Med. 2025. PMID: 39953389 Free PMC article.
References
-
- Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, Della Porta MG, Fenaux P, Gattermann N, Germing U, Jansen JH, Mittelman M, Mufti G, Platzbecker U, Sanz GF, Selleslag D, Skov-Holm M, Stauder R, Symeonidis A, van de Loosdrecht AA, de Witte T, Cazzola M. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943–2964. doi: 10.1182/blood-2013-03-492884. - DOI - PMC - PubMed
-
- Diamantopoulos PT, Kotsianidis I, Symeonidis A, Pappa V, Galanopoulos A, Gogos D, Karakatsanis S, Papadaki H, Palla A, Hatzimichael E, Dimou M, Papageorgiou S, Delimpasis S, Papaioannou M, Papoutselis M, Kourakli A, Tsokanas D, Anagnostopoulos A, Kontos CK, Panayiotidis P, Viniou NA. Chronic myelomonocytic leukemia treated with 5-azacytidine—results from the Hellenic 5-Azacytidine Registry: proposal of a new risk stratification system. Leuk Lymphoma. 2019;60(7):1721–1730. doi: 10.1080/10428194.2018.1540783. - DOI - PubMed
-
- Adès L, Sekeres MA, Wolfromm A, Teichman ML, Tiu RV, Itzykson R, Maciejewski JP, Dreyfus F, List AF, Fenaux P, Komrokji RS. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37(6):609–613. doi: 10.1016/j.leukres.2013.01.004. - DOI - PubMed
-
- Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, Bernal del Castillo T, Al-Ali HK, Martinelli G, Falantes J, Noppeney R, Stone RM, Minden MD, McIntyre H, Songer S, Lucy LM, Beach CL, Döhner H. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

