Disease progression modelling reveals heterogeneity in trajectories of Lewy-type α-synuclein pathology
- PMID: 38879548
- PMCID: PMC11180185
- DOI: 10.1038/s41467-024-49402-x
Disease progression modelling reveals heterogeneity in trajectories of Lewy-type α-synuclein pathology
Abstract
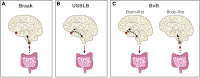
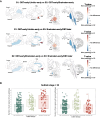
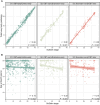
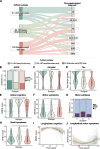
Lewy body (LB) diseases, characterized by the aggregation of misfolded α-synuclein proteins, exhibit notable clinical heterogeneity. This may be due to variations in accumulation patterns of LB neuropathology. Here we apply a data-driven disease progression model to regional neuropathological LB density scores from 814 brain donors with Lewy pathology. We describe three inferred trajectories of LB pathology that are characterized by differing clinicopathological presentation and longitudinal antemortem clinical progression. Most donors (81.9%) show earliest pathology in the olfactory bulb, followed by accumulation in either limbic (60.8%) or brainstem (21.1%) regions. The remaining donors (18.1%) initially exhibit abnormalities in brainstem regions. Early limbic pathology is associated with Alzheimer's disease-associated characteristics while early brainstem pathology is associated with progressive motor impairment and substantial LB pathology outside of the brain. Our data provides evidence for heterogeneity in the temporal spread of LB pathology, possibly explaining some of the clinical disparities observed in Lewy body disease.
© 2024. The Author(s).
Conflict of interest statement
L.E.C. has received research support from GE Healthcare (paid to institution). F.B. acts as a consultant for Biogen-Idec, IXICO, Merck-Serono, Novartis, Combinostics, and Roche. He has received grants, or grants are pending, from the Amyloid Imaging to Prevent Alzheimer’s Disease (AMYPAD) initiative, the Biomedical Research Centre at University College London Hospitals, the Dutch MS Society, ECTRIMS–MAGNIMS, EU-H2020, the Dutch Research Council (NWO), the UK MS Society, and the National Institute for Health Research, University College London. He has received payments for the development of educational presentations from Ixico and his institution from Biogen-Idec and Merck. He is on the editorial board of Radiology, European Neuroradiology, Multiple Sclerosis Journal, and Neurology. Is on the board of directors of Queen Square Analytics. R.O. has received research support from Avid Radiopharmaceuticals, has given lectures in symposia sponsored by GE Healthcare and is an editorial board member of Alzheimer’s Research & Therapy and the European Journal of Nuclear Medicine and Molecular Imaging. O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. T.G.B. is a consultant for Aprinoia Therapeutics, Biogen and Avid Radiopharmaceuticals. A.A. has received, over the last 10 years, honoraria or support for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory or oversight boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Alzheimer’s Disease International, Axovant, AZ Therapies, Biogen, Eisai, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Prothena, Roche/Genentech, Novo Nordisk, Qynapse, Sunovion, Suven, and Synexus. Dr. Atri receives book royalties from Oxford University Press for a medical book on dementia. Dr. Atri receives institutional research grant/contract funding from NIA/NIH 1P30AG072980, NIA/NIH U22AG057437, AZ DHS CTR040636, Washington University St Louis, and Gates Ventures. Dr. Atri’s institution receives/received funding for clinical trial grants, contracts, and projects from government, consortia, foundations and companies for which he serves/served as contracted site-PI. S.H.M. serves as a site co-investigator and receives funding for the PPMI study from the Michael J Fox Foundation. S.H.M. has received research funding from the Arizona Biomedical Research Consortium (ABRC) and International Essential Tremor Foundation (IETF). S.H.M. also has received funding for clinical trial grants, contracts, and projects from government and companies for which S.H.M. serves or has served as contracted site-PI. P.C. has received research support from Lewy Body Dementia association and Arizona Alzheimer’s Consortium (both paid to institution). The remaining authors declare no competing interests.
Figures



Update of
-
Disease progression modelling reveals heterogeneity in trajectories of Lewy-type α-synuclein pathology.bioRxiv [Preprint]. 2023 Dec 7:2023.12.05.569878. doi: 10.1101/2023.12.05.569878. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 15;15(1):5133. doi: 10.1038/s41467-024-49402-x. PMID: 38106128 Free PMC article. Updated. Preprint.
References
-
- Galaz, Z. et al. in Intertwining Graphonomics with Human Movements (eds Cristina Carmona-Duarte, Moises Diaz, Miguel A. Ferrer, & Aythami Morales) 255–268 (Springer International Publishing, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

