Disruption in glutathione metabolism and altered energy production in the liver and kidney after ischemic acute kidney injury in mice
- PMID: 38879688
- PMCID: PMC11180093
- DOI: 10.1038/s41598-024-64586-4
Disruption in glutathione metabolism and altered energy production in the liver and kidney after ischemic acute kidney injury in mice
Abstract
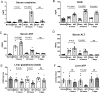
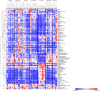
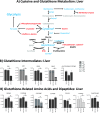
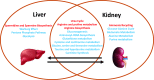
Acute kidney injury (AKI) is a systemic disease that affects energy metabolism in various remote organs in murine models of ischemic AKI. However, AKI-mediated effects in the liver have not been comprehensively assessed. After inducing ischemic AKI in 8-10-week-old, male C57BL/6 mice, mass spectrometry metabolomics revealed that the liver had the most distinct phenotype 24 h after AKI versus 4 h and 7 days. Follow up studies with in vivo [13C6]-glucose tracing on liver and kidney 24 h after AKI revealed 4 major findings: (1) increased flux through glycolysis and the tricarboxylic (TCA) cycle in both kidney and liver; (2) depleted hepatic glutathione levels and its intermediates despite unchanged level of reactive oxygen species, suggesting glutathione consumption exceeds production due to systemic oxidative stress after AKI; (3) hepatic ATP depletion despite unchanged rate of mitochondrial respiration, suggesting increased ATP consumption relative to production; (4) increased hepatic and renal urea cycle intermediates suggesting hypercatabolism and upregulation of the urea cycle independent of impaired renal clearance of nitrogenous waste. Taken together, this is the first study to describe the hepatic metabolome after ischemic AKI in a murine model and demonstrates that there is significant liver-kidney crosstalk after AKI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

