Vectored Thermal Pulsation as a Treatment for Meibomian Gland Dysfunction: A Review Spanning 15 Years
- PMID: 38879718
- PMCID: PMC11246355
- DOI: 10.1007/s40123-024-00976-1
Vectored Thermal Pulsation as a Treatment for Meibomian Gland Dysfunction: A Review Spanning 15 Years
Abstract
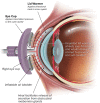
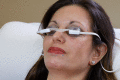
The LipiFlow Thermal Pulsation System received its first marketing clearance for the treatment of meibomian gland dysfunction (MGD) 13 years ago. Since then, the evidence evaluating the effectiveness and safety of LipiFlow as a treatment for MGD has grown significantly. The objective of this comprehensive review was to summarize all clinical reports evaluating the effectiveness and safety of LipiFlow over the past 15 years. The literature was systematically reviewed, and 55 unique articles had subjective (patient-reported outcomes) and objective (meibomian gland function, tear production, and ocular staining) outcomes for extraction. Data were collected from 2101 patients and 3521 eyes treated with LipiFlow. Of these, effectiveness was evaluated in 2041 patients and 3401 eyes, and safety was evaluated in 1448 patients and 2443 eyes. Taken together, the studies demonstrate that a single 12-min treatment with LipiFlow safely improves signs and symptoms of MGD and associated evaporative dry eye disease (DED), and the benefits persist up to 3 years in some cases. The findings are corroborated by multiple meta-analyses and consensus guidelines. While some studies showed that daily eyelid hygiene, warm compress, and/or massage had a similar benefit to a single LipiFlow, these treatments were limited by inconvenience, discomfort, and non-compliance. The majority of studies evaluating safety reported no discomfort or pain associated with LipiFlow treatment, which supports the patient acceptability of LipiFlow therapy. All adverse events (AEs) related to LipiFlow were transient, non-vision-threatening, and did not require treatment. No studies reported serious AEs. The data obtained from 55 studies conducted globally overwhelmingly show that LipiFlow is effective and safe for the treatment of MGD and associated evaporative DED. The conclusions are supported by the diversity of the patient populations (geography, race, disease severity, and diagnosis), the large population treated with LipiFlow, the meta-analyses, and that this review analyzed all published clinical studies to date.
Keywords: Dry eye; LipiFlow; Meibomian gland dysfunction; Ocular surface disease; Vectored thermal pulsation system.
© 2024. The Author(s).
Conflict of interest statement
Heather S. Oliff declares that she has no competing interests. Caroline A. Blackie and David Murakami are employees of Johnson & Johnson Surgical Vision. Eric Donnenfeld is a consultant to Johnson & Johnson Surgical Vision.
Figures
Similar articles
-
Is a thermal pulsation system (LipiFlow) effective as a standalone treatment for meibomian gland dysfunction and dry eye? A systematic review and meta-analysis.Ther Adv Ophthalmol. 2025 May 10;17:25158414251338775. doi: 10.1177/25158414251338775. eCollection 2025 Jan-Dec. Ther Adv Ophthalmol. 2025. PMID: 40352434 Free PMC article.
-
LipiFlow for the treatment of dry eye disease.Cochrane Database Syst Rev. 2024 Feb 5;2(2):CD015448. doi: 10.1002/14651858.CD015448.pub2. Cochrane Database Syst Rev. 2024. PMID: 38314898 Free PMC article.
-
Efficacy and Safety evaluation of a single thermal pulsation system treatment (Lipiflow®) on meibomian gland dysfunction: a randomized controlled clinical trial.Int Ophthalmol. 2023 Apr;43(4):1175-1184. doi: 10.1007/s10792-022-02516-x. Epub 2022 Sep 16. Int Ophthalmol. 2023. PMID: 36112256 Clinical Trial.
-
Preoperative Management of MGD with Vectored Thermal Pulsation before Cataract Surgery: A Prospective, Controlled Clinical Trial.Semin Ophthalmol. 2021 Feb 17;36(1-2):2-8. doi: 10.1080/08820538.2021.1881567. Epub 2021 Feb 15. Semin Ophthalmol. 2021. PMID: 33587674 Clinical Trial.
-
Thermal Pulsation in the Management of Meibomian Gland Dysfunction and Dry Eye: A Report by the American Academy of Ophthalmology.Ophthalmology. 2023 Dec;130(12):1336-1341. doi: 10.1016/j.ophtha.2023.07.009. Epub 2023 Aug 27. Ophthalmology. 2023. PMID: 37642619 Review.
Cited by
-
Is a thermal pulsation system (LipiFlow) effective as a standalone treatment for meibomian gland dysfunction and dry eye? A systematic review and meta-analysis.Ther Adv Ophthalmol. 2025 May 10;17:25158414251338775. doi: 10.1177/25158414251338775. eCollection 2025 Jan-Dec. Ther Adv Ophthalmol. 2025. PMID: 40352434 Free PMC article.
-
Varenicline Nasal Spray for the Treatment of Dry Eye Disease Following Corneal Collagen Crosslinking.Ophthalmol Ther. 2025 May;14(5):959-968. doi: 10.1007/s40123-025-01118-x. Epub 2025 Mar 15. Ophthalmol Ther. 2025. PMID: 40088395 Free PMC article.
-
Optimizing Diagnosis and Management of Dry Eye Disease: A Practical Framework for Hong Kong.Ophthalmol Ther. 2025 May;14(5):815-833. doi: 10.1007/s40123-025-01129-8. Epub 2025 Mar 26. Ophthalmol Ther. 2025. PMID: 40138170 Free PMC article.
References
-
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93-107. - PubMed
-
- Blackie CA, Folly E, Ruppenkamp J, Holy C. Prevalence of meibomian gland dysfunction—a systematic review and analysis of published evidence. IOVS. 2019;60(9):2736.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

