Building a novel TRUCK by harnessing the endogenous IFN-gamma promoter for cytokine expression
- PMID: 38879754
- PMCID: PMC11405158
- DOI: 10.1016/j.ymthe.2024.06.017
Building a novel TRUCK by harnessing the endogenous IFN-gamma promoter for cytokine expression
Abstract
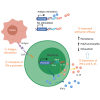
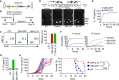
Despite the remarkable success of chimeric antigen receptor (CAR) T therapy in hematological malignancies, its efficacy in solid tumors remains limited. Cytokine-engineered CAR T cells offer a promising avenue, yet their clinical translation is hindered by the risks associated with constitutive cytokine expression. In this proof-of-concept study, we leverage the endogenous interferon (IFN)-γ promoter for transgenic interleukin (IL)-15 expression. We demonstrate that IFN-γ expression is tightly regulated by T cell receptor signaling. By introducing an internal ribosome entry site IL15 into the 3' UTR of the IFN-γ gene via homology directed repair-mediated knock-in, we confirm that IL-15 expression can co-express with IFN-γ in an antigen stimulation-dependent manner. Importantly, the insertion of transgenes does not compromise endogenous IFN-γ expression. In vitro and in vivo data demonstrate that IL-15 driven by the IFN-γ promoter dramatically improves CAR T cells' antitumor activity, suggesting the effectiveness of IL-15 expression. Last, as a part of our efforts toward clinical translation, we have developed an innovative two-gene knock-in approach. This approach enables the simultaneous integration of CAR and IL-15 genes into TRAC and IFN-γ gene loci using a single AAV vector. CAR T cells engineered to express IL-15 using this approach demonstrate enhanced antitumor efficacy. Overall, our study underscores the feasibility of utilizing endogenous promoters for transgenic cytokines expression in CAR T cells.
Keywords: CAR; IFN-γ; IL-15; antitumor activity; chimeric antigen receptor; endogenous promoter; interferon-γ; interleukin-15.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The study was funded by Shenzhen Celconta Life Science Co., Ltd. Two patents related to this study have been filed, and Shenzhen Celconta Life Science Co., Ltd. holds the right of the patents.
Figures





References
-
- Lee E.H.J., Murad J.P., Christian L., Gibson J., Yamaguchi Y., Cullen C., Gumber D., Park A.K., Young C., Monroy I., et al. Antigen-dependent IL-12 signaling in CAR T cells promotes regional to systemic disease targeting. Nat. Commun. 2023;14:4737. doi: 10.1038/s41467-023-40115-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

