Relationship between reasons for intermittent missing patient-reported outcomes data and missing data mechanisms
- PMID: 38879861
- PMCID: PMC11390842
- DOI: 10.1007/s11136-024-03707-y
Relationship between reasons for intermittent missing patient-reported outcomes data and missing data mechanisms
Abstract
Purpose: Non-response (NR) to patient-reported outcome (PRO) questionnaires may cause bias if not handled appropriately. Collecting reasons for NR is recommended, but how reasons for NR are related to missing data mechanisms remains unexplored. We aimed to explore this relationship for intermittent NRs.
Methods: Patients with multiple myeloma completed validated PRO questionnaires at enrolment and 12 follow-up time-points. NR was defined as non-completion of a follow-up assessment within seven days, which triggered contact with the patient, recording the reason for missingness and an invitation to complete the questionnaire (denoted "salvage response"). Mean differences between salvage and previous on-time scores were estimated for groups defined by reasons for NR using linear regression with clustered standard errors. Statistically significant mean differences larger than minimal important difference thresholds were interpreted as "missing not at random" (MNAR) mechanism (i.e. assumed to be related to declining health), and the remainder interpreted as aligned with "missing completely at random" (MCAR) mechanism (i.e. assumed unrelated to changes in health).
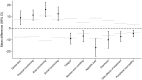
Results: Most (7228/7534 (96%)) follow-up questionnaires were completed; 11% (802/7534) were salvage responses. Mean salvage scores were compared to previous on-time scores by reason: those due to hospital admission, mental or physical reasons were worse in 10/22 PRO domains; those due to technical difficulties/procedural errors were no different in 21/22 PRO domains; and those due to overlooked/forgotten or other/unspecified reasons were no different in any domains.
Conclusion: Intermittent NRs due to hospital admission, mental or physical reasons were aligned with MNAR mechanism for nearly half of PRO domains, while intermittent NRs due to technical difficulties/procedural errors or other/unspecified reasons generally were aligned with MCAR mechanism.
Keywords: Missing data; Missing data mechanism; Multiple myeloma; Patient-reported outcomes; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
References
-
- Ahmed, S., Berzon, R. A., Revicki, D. A., Lenderking, W. R., Moinpour, C. M., Basch, E., Reeve, B. B., & Wu, A. W. (2012). The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Medical Care,50(12), 1060–1070. 10.1097/MLR.0b013e318268aaff 10.1097/MLR.0b013e318268aaff - DOI - PubMed
-
- Fayers, P. M., & Machin, D. (2016). Quality of life: The assessment, analysis, and reporting of patient-reported outcomes (3rd ed.). Wiley.
-
- Fairclough, D. L. (2010). Design and analysis of quality of life studies in clinical trials. Chapman & Hall/CRC interdisciplinary statistics series (2nd ed.). Chapman & Hall/CRC.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

