Spatial distribution of Mycobacterium tuberculosis mRNA and secreted antigens in acid-fast negative human antemortem and resected tissue
- PMID: 38880068
- PMCID: PMC11233921
- DOI: 10.1016/j.ebiom.2024.105196
Spatial distribution of Mycobacterium tuberculosis mRNA and secreted antigens in acid-fast negative human antemortem and resected tissue
Abstract
Background: The ability to detect evidence of Mycobacterium tuberculosis (Mtb) infection within human tissues is critical to the study of Mtb physiology, tropism, and spatial distribution within TB lesions. The capacity of the widely-used Ziehl-Neelsen (ZN) staining method for identifying Mtb acid-fast bacilli (AFB) in tissue is highly variable, which can limit detection of Mtb bacilli for research and diagnostic purposes. Here, we sought to circumvent these limitations via detection of Mtb mRNA and secreted antigens in human tuberculous tissue.
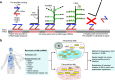
Methods: We adapted RNAscope, an RNA in situ hybridisation (RISH) technique, to detect Mtb mRNA in ante- and postmortem human TB tissues and developed a dual ZN/immunohistochemistry staining approach to identify AFB and bacilli producing antigen 85B (Ag85B).
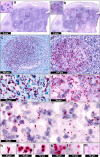
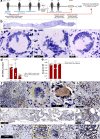
Findings: We identified Mtb mRNA within intact and disintegrating bacilli as well as extrabacillary mRNA. Mtb mRNA was distributed zonally within necrotic and non-necrotic granulomas. We also found Mtb mRNA within, and adjacent to, necrotic granulomas in ZN-negative lung tissue and in Ag85B-positive bronchiolar epithelium. Intriguingly, we observed accumulation of Mtb mRNA and Ag85B in the cytoplasm of host cells. Notably, many AFB were negative for Ag85B staining. Mtb mRNA was observed in ZN-negative antemortem lymph node biopsies.
Interpretation: RNAscope and dual ZN/immunohistochemistry staining are well-suited for identifying subsets of intact Mtb and/or bacillary remnants in human tissue. RNAscope can identify Mtb mRNA in ZN-negative tissues from patients with TB and may have diagnostic potential in complex TB cases.
Funding: Wellcome Leap Delta Tissue Program, Wellcome Strategic Core Award, the National Institutes of Health (NIH, USA), the Mary Heersink Institute for Global Health at UAB, the UAB Heersink School of Medicine.
Keywords: Antigen; Diagnosis; RNAscope; Tuberculosis; Ziehl-Neelsen.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no competing interests or disclosures.
Figures




References
-
- Goletti D., Delogu G., Matteelli A., Migliori G.B. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis. 2022;124(Suppl 1):S12–S19. - PubMed
-
- Barcellini L., Borroni E., Brown J., et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J. 2016;48(5):1411–1419. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

