Primary Ciliary Dyskinesia in Adult Bronchiectasis: Data from the German Bronchiectasis Registry PROGNOSIS
- PMID: 38880279
- PMCID: PMC11562653
- DOI: 10.1016/j.chest.2024.05.023
Primary Ciliary Dyskinesia in Adult Bronchiectasis: Data from the German Bronchiectasis Registry PROGNOSIS
Abstract
Background: Primary ciliary dyskinesia (PCD) is a rare genetic disorder caused by the malfunction of motile cilia and a specific etiology of adult bronchiectasis of unknown prevalence. A better understanding of the clinical phenotype of adults with PCD is needed to identify individuals for referral to diagnostic testing.
Research question: What is the frequency of PCD among adults with bronchiectasis; how do people with PCD differ from those with other etiologies; and which clinical characteristics are independently associated with PCD?
Study design and methods: We investigated the proportion of PCD among the participants of the Prospective German Non-CF-Bronchiectasis Registry (PROGNOSIS) study; applied multiple imputation to account for missing data in 64 (FEV1), 58 (breathlessness), 26 (pulmonary exacerbations), and two patients (BMI), respectively; and identified predictive variables from baseline data using multivariate logistic regression analysis.
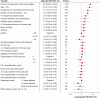
Results: We consecutively recruited 1,000 patients from 38 centers across all levels of the German health care system. Overall, PCD was the fifth most common etiology of bronchiectasis in 87 patients (9%) after idiopathic, postinfective, COPD, and asthma. People with PCD showed a distinct clinical phenotype. In multivariate regression analysis, the chance of PCD being the etiology of bronchiectasis increased with the presence of upper airway disease (chronic rhinosinusitis and/or nasal polyps; adjusted OR [aOR], 6.3; 95% CI, 3.3-11.9; P < .001), age < 53 years (aOR, 5.3; 95% CI, 2.7-10.4; P < .001), radiologic involvement of any middle and lower lobe (aOR, 3.7; 95% CI, 1.3-10.8; P = .016), duration of bronchiectasis > 15 years (aOR, 3.6; 95% CI, 1.9-6.9; P < .001), and a history of Pseudomonas aeruginosa isolation from respiratory specimen (aOR, 2.4; 95% CI, 1.3-4.5; P = .007).
Interpretation: Within our nationally representative cohort, PCD was a common etiology of bronchiectasis. We identified few easy-to-assess phenotypic features, which may promote awareness for PCD among adults with bronchiectasis.
Clinical trial registration: ClinicalTrials.gov; No.: NCT02574143; URL: www.
Clinicaltrials: gov.
Keywords: Kartagener syndrome; bronchiectasis; phenotype; primary ciliary dyskinesia; registries.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: I. P. reports grants from COFONI-2FF4 and COFONI-6LZF23 by the Ministry of Science and Culture of Lower Saxony paid to her institution and personal lecture fees from AstraZeneca and Boehringer Ingelheim. S. S. reports fees for clinical trial participation from Celtaxsys, Corbus, Galapagos, Insmed, Proteostasis, and Vertex Pharmaceuticals paid to his institution; and personal fees for consulting and lectures from Boehringer Ingelheim, Insmed, and Vertex Pharmaceuticals. S. P. A. reports personal consulting fees from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, and GlaxoSmithKline. A. G. reports personal fees for consulting, advisory board participation, and lectures from Boehringer Ingelheim and GlaxoSmithKline. A. S. reports personal fees for consulting and lectures from Ethris, Insmed, Spirovant, and Translate Bio; and is involved in the European Respiratory Society (ERS) Clinical Research Collaborations AMR-Lung, BEAT-PCD, and EMBARC. S. W. reports fees for clinical trial participation from Insmed paid to her institution and personal honoraria from Vertex Pharmaceuticals. P. M. reports fees for clinical trial participation from Boehringer Ingelheim and Insmed paid to his institution; reports personal fees for lectures from AstraZeneca, MAÄF eV, ResMed, and streamedup! GmbH; reports travel support from the German Society for Internal Medicine (DGIM), CSL Behring, and Insmed; and is honorary co-chair of the German Bronchiectasis Registry PROGNOSIS. J. R. reports grants from the German Center for Lung Research (DZL), the German Center for Infection Research (DZIF), the Federal Ministry of Education and Research (BMBF), the Federal Ministry of Health (BMG), Novartis, and Insmed paid to her institution; reports personal fees for consulting or advisory board participation and honoraria for lectures from AstraZeneca, Brahms GmbH, ERS, Grifols, Insmed, MedUpdate, MSD, Pfizer, Shionogi, and streamedup! GmbH; and is honorary co-chair of the German Bronchiectasis Registry PROGNOSIS and chair of the Respiratory Infections and Tuberculosis Assembly of the German Respiratory Society (DGP). F. C. R. reports grants from the German Center for Lung Research (DZL), the German Center for Infection Research (DZIF), IMI (EU/EFPIA), and the Innovative Medicines Initiative (IMI) and EFPIA companies under the European Commission funded project, iABC [Grant 115721], Novartis, and Insmed Germany paid to his institution; reports personal fees for consulting or advisory board participation and honoraria for lectures from Parion, Grifols, Zambon, Insmed, Helmholtz-Zentrum für Infektionsforschung, i!DE Werbeagentur GmbH, Interkongress GmbH, streamedup! GmbH, AstraZeneca, Insmed, Shionogi, and Grifols; reports travel support from the German Kartagener Syndrome and Primary Ciliary Dyskinesia patient advocacy group, which he serves as the unpaid co-speaker of its medical advisory board; and is honorary co-chair of the German Bronchiectasis Registry PROGNOSIS, a member of the steering committee of the European Bronchiectasis Registry EMBARC, and a member of the Protocol Review Committee of the PCD-Clinical Trials Network. None declared (R. E., U. S., B. O. S.).
Figures



References
-
- Ringshausen F.C., Rademacher J., Pink I., et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6) - PubMed
-
- Chalmers J.D., Polverino E., Crichton M.L., et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC) Lancet Respir Med. 2023;11(7):637–649. - PubMed
-
- Aliberti S., Goeminne P.C., O'Donnell A.E., et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med. 2022;10(3):298–306. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

