DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease
- PMID: 38880789
- PMCID: PMC11180664
- DOI: 10.1038/s41392-024-01881-6
DNA of neutrophil extracellular traps promote NF-κB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease
Abstract
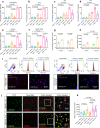
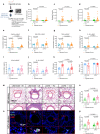
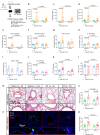
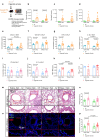
Chronic obstructive pulmonary disease (COPD) is characterised by persistent airway inflammation even after cigarette smoking cessation. Neutrophil extracellular traps (NETs) have been implicated in COPD severity and acute airway inflammation induced by short-term cigarette smoke (CS). However, whether and how NETs contribute to sustained airway inflammation in COPD remain unclear. This study aimed to elucidate the immunoregulatory mechanism of NETs in COPD, employing human neutrophils, airway epithelial cells (AECs), dendritic cells (DCs), and a long-term CS-induced COPD mouse model, alongside cyclic guanosine monophosphate-adenosine monophosphate synthase and toll-like receptor 9 knockout mice (cGAS--/-, TLR9-/-); Additionally, bronchoalveolar lavage fluid (BALF) of COPD patients was examined. Neutrophils from COPD patients released greater cigarette smoke extract (CSE)-induced NETs (CSE-NETs) due to mitochondrial respiratory chain dysfunction. These CSE-NETs, containing oxidatively-damaged DNA (NETs-DNA), promoted AECs proliferation, nuclear factor kappa B (NF-κB) activation, NF-κB-dependent cytokines and type-I interferons production, and DC maturation, which were ameliorated/reversed by silencing/inhibition of cGAS/TLR9. In the COPD mouse model, blocking NETs-DNA-sensing via cGAS-/- and TLR9-/- mice, inhibiting NETosis using mitoTEMPO, and degrading NETs-DNA with DNase-I, respectively, reduced NETs infiltrations, airway inflammation, NF-κB activation and NF-κB-dependent cytokines, but not type-I interferons due to IFN-α/β receptor degradation. Elevated NETs components (myeloperoxidase and neutrophil elastase activity) in BALF of COPD smokers correlated with disease severity and NF-κB-dependent cytokine levels, but not type-I interferon levels. In conclusion, NETs-DNA promotes NF-κB-dependent autoimmunity via cGAS/TLR9 in long-term CS exposure-induced COPD. Therefore, targeting NETs-DNA and cGAS/TLR9 emerges as a potential strategy to alleviate persistent airway inflammation in COPD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. http://goldcopd.org/ (2024).
Publication types
MeSH terms
Substances
Grants and funding
- 81830001/National Natural Science Foundation of China (National Science Foundation of China)
- 81670038/National Natural Science Foundation of China (National Science Foundation of China)
- 81800015/National Natural Science Foundation of China (National Science Foundation of China)
- 31871157/National Natural Science Foundation of China (National Science Foundation of China)
- 2019T120851/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

