Revealing molecular determinants governing mambalgin-3 pharmacology at acid-sensing ion channel 1 variants
- PMID: 38880807
- PMCID: PMC11335189
- DOI: 10.1007/s00018-024-05276-2
Revealing molecular determinants governing mambalgin-3 pharmacology at acid-sensing ion channel 1 variants
Abstract
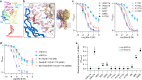
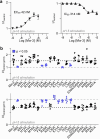
Acid-sensing ion channels (ASICs) are trimeric proton-gated cation channels that play a role in neurotransmission and pain sensation. The snake venom-derived peptides, mambalgins, exhibit potent analgesic effects in rodents by inhibiting central ASIC1a and peripheral ASIC1b. Despite their distinct species- and subtype-dependent pharmacology, previous structure-function studies have focussed on the mambalgin interaction with ASIC1a. Currently, the specific channel residues responsible for this pharmacological profile, and the mambalgin pharmacophore at ASIC1b remain unknown. Here we identify non-conserved residues at the ASIC1 subunit interface that drive differences in the mambalgin pharmacology from rat ASIC1a to ASIC1b, some of which likely do not make peptide binding interactions. Additionally, an amino acid variation below the core binding site explains potency differences between rat and human ASIC1. Two regions within the palm domain, which contribute to subtype-dependent effects for mambalgins, play key roles in ASIC gating, consistent with subtype-specific differences in the peptides mechanism. Lastly, there is a shared primary mambalgin pharmacophore for ASIC1a and ASIC1b activity, with certain peripheral peptide residues showing variant-specific significance for potency. Through our broad mutagenesis studies across various species and subtype variants, we gain a more comprehensive understanding of the pharmacophore and the intricate molecular interactions that underlie ligand specificity. These insights pave the way for the development of more potent and targeted peptide analogues required to advance our understating of human ASIC1 function and its role in disease.
Keywords: ASIC; Allosteric modulation; Electrophysiology; Gating modifier; Ligand selectivity; Protein-protein interaction; Specificity; Venom peptide.
© 2024. The Author(s).
Conflict of interest statement
Author LDR is a co-inventor on a patent (US 10,485,847) for the use of the ASIC inhibitory peptide Hi1a as a neuroprotective agent. All other authors have no relevant financial or non-financial interests to disclose.
Figures






Similar articles
-
Mambalgin-1 pain-relieving peptide locks the hinge between α4 and α5 helices to inhibit rat acid-sensing ion channel 1a.Neuropharmacology. 2021 Mar 1;185:108453. doi: 10.1016/j.neuropharm.2021.108453. Epub 2021 Jan 12. Neuropharmacology. 2021. PMID: 33450275
-
The modulation of acid-sensing ion channel 1 by PcTx1 is pH-, subtype- and species-dependent: Importance of interactions at the channel subunit interface and potential for engineering selective analogues.Biochem Pharmacol. 2019 May;163:381-390. doi: 10.1016/j.bcp.2019.03.004. Epub 2019 Mar 5. Biochem Pharmacol. 2019. PMID: 30849303
-
Mambalgin-3 potentiates human acid-sensing ion channel 1b under mild to moderate acidosis: Implications as an analgesic lead.Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2021581118. doi: 10.1073/pnas.2021581118. Proc Natl Acad Sci U S A. 2021. PMID: 33602819 Free PMC article.
-
Mechanisms of Action of the Peptide Toxins Targeting Human and Rodent Acid-Sensing Ion Channels and Relevance to Their In Vivo Analgesic Effects.Toxins (Basel). 2022 Oct 17;14(10):709. doi: 10.3390/toxins14100709. Toxins (Basel). 2022. PMID: 36287977 Free PMC article. Review.
-
Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms.Neuropharmacology. 2017 Dec;127:173-184. doi: 10.1016/j.neuropharm.2017.04.042. Epub 2017 Apr 27. Neuropharmacology. 2017. PMID: 28457973 Review.
Cited by
-
Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin.Toxins (Basel). 2025 Feb 21;17(3):101. doi: 10.3390/toxins17030101. Toxins (Basel). 2025. PMID: 40137874 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

