Disturbed glycolipid metabolism activates CXCL13-CXCR5 axis in senescent TSCs to promote heterotopic ossification
- PMID: 38880863
- PMCID: PMC11335191
- DOI: 10.1007/s00018-024-05302-3
Disturbed glycolipid metabolism activates CXCL13-CXCR5 axis in senescent TSCs to promote heterotopic ossification
Abstract
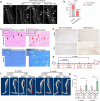
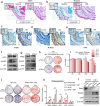
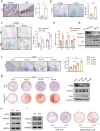
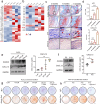
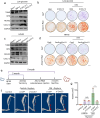
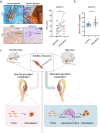
Heterotopic ossification (HO) occurs as a common complication after injury, while its risk factor and mechanism remain unclear, which restricts the development of pharmacological treatment. Clinical research suggests that diabetes mellitus (DM) patients are prone to developing HO in the tendon, but solid evidence and mechanical research are still needed. Here, we combined the clinical samples and the DM mice model to identify that disordered glycolipid metabolism aggravates the senescence of tendon-derived stem cells (TSCs) and promotes osteogenic differentiation. Then, combining the RNA-seq results of the aging tendon, we detected the abnormally activated autocrine CXCL13-CXCR5 axis in TSCs cultured in a high fat, high glucose (HFHG) environment and also in the aged tendon. Genetic inhibition of CXCL13 successfully alleviated HO formation in DM mice, providing a potential therapeutic target for suppressing HO formation in DM patients after trauma or surgery.
Keywords: CXCL13; Cellular senescence; Glycolipid metabolism; Heterotopic ossification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

