Immune Mechanisms in Hypertension
- PMID: 38881474
- PMCID: PMC11254551
- DOI: 10.1161/HYPERTENSIONAHA.124.21355
Immune Mechanisms in Hypertension
Abstract
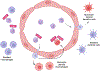
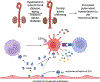
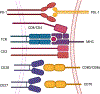
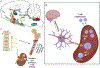
It is now apparent that immune mediators including complement, cytokines, and cells of the innate and adaptive immune system contribute not only to blood pressure elevation but also to the target organ damage that occurs in response to stimuli like high salt, aldosterone, angiotensin II, and sympathetic outflow. Alterations of vascular hemodynamic factors, including microvascular pulsatility and shear forces, lead to vascular release of mediators that affect myeloid cells to become potent antigen-presenting cells and promote T-cell activation. Research in the past 2 decades has defined specific biochemical and molecular pathways that are engaged by these stimuli and an emerging paradigm is these not only lead to immune activation, but that products of immune cells, including cytokines, reactive oxygen species, and metalloproteinases act on target cells to further raise blood pressure in a feed-forward fashion. In this review, we will discuss these molecular and pathophysiological events and discuss clinical interventions that might prove effective in quelling this inflammatory process in hypertension and related cardiovascular diseases.
Keywords: autoimmunity; cytokines; dendritic cells; inflammation; neuroimmune.
Conflict of interest statement
None.
Figures



References
-
- Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, Saito S, Matsumoto K, Mugishima H. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004:42–47: 10.1161/01.HYP.0000129540.83284.ca - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

