Effect of direct-acting antivirals on disease burden of hepatitis C virus infection in South Korea in 2007-2021: a nationwide, multicentre, retrospective cohort study
- PMID: 38881570
- PMCID: PMC11176940
- DOI: 10.1016/j.eclinm.2024.102671
Effect of direct-acting antivirals on disease burden of hepatitis C virus infection in South Korea in 2007-2021: a nationwide, multicentre, retrospective cohort study
Abstract
Background: It is unclear whether direct-acting antivirals (DAAs) treatment improves the disease burden in hepatitis C virus (HCV) infection. This study aimed to investigate the effect of DAA treatment on the reduction of disease burden in patients with HCV infection using individual participant data.
Methods: This nationwide multicentre retrospective cohort study recruited patients with HCV infection from 29 tertiary institutions in South Korea. The data collection was done from medical records in each institution. The study included the untreated patients and the DAAs-treated patients and excluded those with a history of interferon-based treatments. Disease burden was the primary outcome, as represented by disability-adjusted life years (DALYs). Improvement in fibrosis after DAA treatment was assessed using APRI, FIB-4 index, and liver stiffness (LS) as assessed by transient elastography. Clinical outcomes were hepatocellular carcinoma (HCC), decompensation, and mortality.
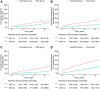
Findings: Between January 1, 2007, and February 17, 2022, data from 11,725 patients with HCV infection, 8464 (72%) of whom were treated with DAAs, were analysed. DAA treatment significantly improved APRI- (median 0.64 [interquartile range (IQR), 0.35-1.31]-0.33 [0.23-0.52], p < 0.0001), FIB-4- (median 2.42 [IQR, 1.48-4.40]-1.93 [1.31-2.97], p < 0.0001), and liver LS-based fibrosis (median 7.4 [IQR, 5.3-12.3]-6.2 [4.6-10.2] kPa, p < 0.0001). During the median follow-up period of 27.5 months (IQR, 10.6-52.4), 469 patients died (4.0%), 586 (5.0%) developed HCC, and 580 (4.9%) developed decompensation. The APRI-based DALY estimate was significantly lower in the DAA group than in the untreated group (median 4.55 vs. 5.14 years, p < 0.0001), as was the FIB-4-based DALY estimate (median 5.43 [IQR, 3.00-6.44] vs. 5.79 [3.85-8.07] years, p < 0.0001). The differences between the untreated and DAA groups were greatest in patients aged 40-60 years. In multivariable analyses, the DAA group had a significantly reduced risk of HCC, decompensation, and mortality compared with the untreated group (hazard ratios: 0.41 [95% confidence interval (CI), 0.34-0.48], 0.31 [95% CI, 0.30-0.38], and 0.22 [95% CI, 0.17-0.27], respectively; p < 0.0001).
Interpretation: Our findings suggest that DAA treatment is associated with the improvement of liver-related outcomes and a reduction of liver fibrosis-based disease burden in patients with HCV infection. However, further studies using liver biopsy are needed to clarify the effect of DAA treatment on the reduction in the exact fibrosis-based disease burden beyond noninvasive tests.
Funding: The Korea Disease Control and Prevention Agency.
Keywords: Direct-acting antiviral; Disease burden; Hepatitis C virus; Liver fibrosis.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
All authors declare no financial or non-financial competing interests.
Figures

Similar articles
-
Liver stiffness reduction and serum fibrosis score improvement in HIV/hepatitis C virus-coinfected patients treated with direct-acting antivirals.HIV Med. 2018 Jun 28. doi: 10.1111/hiv.12632. Online ahead of print. HIV Med. 2018. PMID: 29953713
-
Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients.J Hepatol. 2019 Aug;71(2):265-273. doi: 10.1016/j.jhep.2019.03.027. Epub 2019 Apr 6. J Hepatol. 2019. PMID: 30959157
-
Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index.Liver Int. 2017 Mar;37(3):369-376. doi: 10.1111/liv.13256. Epub 2016 Nov 3. Liver Int. 2017. PMID: 27678216
-
AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review.Gastroenterology. 2019 Jun;156(8):2149-2157. doi: 10.1053/j.gastro.2019.02.046. Epub 2019 Mar 13. Gastroenterology. 2019. PMID: 30878469 Free PMC article. Review.
-
Impact of direct-acting antiviral regimens on hepatic and extrahepatic manifestations of hepatitis C virus infection.World J Hepatol. 2022 Jun 27;14(6):1053-1073. doi: 10.4254/wjh.v14.i6.1053. World J Hepatol. 2022. PMID: 35978668 Free PMC article. Review.
Cited by
-
Hepatocellular carcinoma in Korea: an analysis of the 2016-2018 Korean Nationwide Cancer Registry.J Liver Cancer. 2025 Mar;25(1):109-122. doi: 10.17998/jlc.2025.02.20. Epub 2025 Mar 4. J Liver Cancer. 2025. PMID: 40033636 Free PMC article.
-
Non-invasive prediction of post-sustained virological response hepatocellular carcinoma in hepatitis C virus: A systematic review and meta-analysis.Clin Mol Hepatol. 2024 Sep;30(Suppl):S172-S185. doi: 10.3350/cmh.2024.0262. Epub 2024 Aug 12. Clin Mol Hepatol. 2024. PMID: 39134075 Free PMC article.
-
The Malignant Transformation of Viral Hepatitis to Hepatocellular Carcinoma: Mechanisms and Interventions.MedComm (2020). 2025 Mar 8;6(3):e70121. doi: 10.1002/mco2.70121. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40060195 Free PMC article. Review.
-
Viral oncogenesis in cancer: from mechanisms to therapeutics.Signal Transduct Target Ther. 2025 May 12;10(1):151. doi: 10.1038/s41392-025-02197-9. Signal Transduct Target Ther. 2025. PMID: 40350456 Free PMC article. Review.
-
Real-World Treatment Efficacy and Safety Profile of Sofosbuvir- and Velpatasvir-Based HCV Treatment in South Korea: Multicenter Prospective Study.Viruses. 2025 Jul 4;17(7):949. doi: 10.3390/v17070949. Viruses. 2025. PMID: 40733566 Free PMC article.
References
-
- World Health Organization . Accountability for the global health sector strategies 2016-2021: actions for impact. 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021.https://apps.who.int/iris/rest/bitstreams/1348210/retrieve
-
- Sahakyan Y., Lee-Kim V., Bremner K.E., Bielecki J.M., Krahn M.D. Impact of direct-acting antiviral regimens on mortality and morbidity outcomes in patients with chronic hepatitis c: systematic review and meta-analysis. J Viral Hepat. 2021;28(5):739–754. - PubMed
-
- Lens S., Alvarado-Tapias E., Marino Z., et al. Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis. Gastroenterology. 2017;153(5):1273–12783.e1. - PubMed
LinkOut - more resources
Full Text Sources

