Dose-Dependent Progression of Chorioretinal Atrophy at the Injection Site After Subretinal Injection of rAAV2/8 in Nonhuman Primates
- PMID: 38881604
- PMCID: PMC11179412
- DOI: 10.1016/j.xops.2024.100516
Dose-Dependent Progression of Chorioretinal Atrophy at the Injection Site After Subretinal Injection of rAAV2/8 in Nonhuman Primates
Abstract
Objective: Progressive retinal atrophy has been described after subretinal gene therapy utilizing the adeno-associated virus (AAV) vector platform. To elucidate whether this atrophy is a consequence of inherent properties of AAV, or if it is related to the surgical trauma of subretinal delivery, we analyzed data from an Investigational New Drug-enabling study for PDE6A gene therapy in nonhuman primates.
Design: Animal study (nonhuman primates), retrospective data analysis.
Subjects: Forty eyes of 30 healthy nonhuman primates (macaca fascicularis) were included in the analysis. Two AAV dose levels (low: 1x10E11, high: 1x10E12) were compared with sham injection (balanced saline solution; BSS). Twenty untreated eyes were not analyzed.
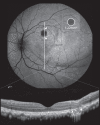
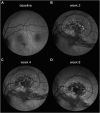
Methods: Animals were treated with a sutureless 23G vitrectomy and single subretinal injections of AAV.PDE6A and/or BSS. The follow-up period was 12 weeks. Atrophy development was followed using fundus autofluorescence (AF), OCT, fluorescence angiography, and indocyanine green angiography.
Main outcome measures: Area [mm2] of retinal pigment epithelium atrophy on AF. Presence of outer retinal atrophy on optical coherence tomography. Area [mm2] of hyperfluorescence in fluorescence angiography and hypofluorescence in indocyanine green angiography.
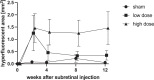
Results: Progressive atrophy at the injection site developed in 54% of high-dose-treated, 27% of low-dose-treated, and 0% of sham-treated eyes. At the end of observation, the mean ± SD area of atrophy in AF was 1.19 ± 1.75 mm2, 0.25 ± 0.50 mm2, and 0.0 ± 0.0 mm2, respectively (sham × high dose: P = 0.01). Atrophic lesions in AF (P = 0.01) and fluorescence angiography (P = 0.02) were significantly larger in high-dose-treated eyes, compared with sham-treated eyes. Rate of progression in high-dose-treated eyes was 4.1× higher compared with low-dose-treated eyes.
Conclusion: Subretinal injection of AAV.PDE6A induced dose-dependent, progressive retinal atrophy at the site of injection. Findings from multimodal imaging were in line with focal, transient inflammation within the retina and choroid and secondary atrophy. Atrophic changes after gene therapy with AAV-based vector systems are not primarily due to surgical trauma and increase with the dose given.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: AAV; atrophy; gene therapy; inflammation; nonhuman primates.
© 2024 Published by Elsevier Inc. on behalf of the American Academy of Ophthalmology.
Figures



References
-
- Reichel F.F., Seitz I., Wozar F., et al. Development of retinal atrophy after subretinal gene therapy with voretigene neparvovec. Br J Ophthalmol. 2023;107:1331–1335. - PubMed
-
- Bucher K., Rodríguez-Bocanegra E., Dauletbekov D., Fischer M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors - implications for treatment success and safety. Prog Retin Eye Res. 2021;83 - PubMed
-
- Kessel L., Christensen U.C., Klemp K. Inflammation after voretigene neparvovec administration in patients with RPE65-related retinal dystrophy. Ophthalmology. 2022;129:1287–1293. - PubMed
LinkOut - more resources
Full Text Sources

