Research protocol and recruitment redesign of a study of pregnant women and their infants during the COVID-19 pandemic: Childhood Allergy and the NeOnatal Environment (CANOE)
- PMID: 38881739
- PMCID: PMC11179080
- DOI: 10.1016/j.jacig.2024.100270
Research protocol and recruitment redesign of a study of pregnant women and their infants during the COVID-19 pandemic: Childhood Allergy and the NeOnatal Environment (CANOE)
Abstract
Background: Recruitment for research studies is a challenging endeavor that was further complicated by the coronavirus disease 2019 pandemic. We launched a new multicenter birth cohort, Childhood Allergy and the NeOnatal Environment (CANOE), supported by the National Institutes of Health in January 2020 across 4 sites. Although the pandemic temporarily halted clinical research, we restructured the study and instituted novel recruitment methods that we hypothesized would enable brisk enrollment when research activities resumed.
Objective: We sought to develop protocol modifications and recruitment methods that promote successful recruitment of diverse populations in clinical research despite a global pandemic.
Methods: Even though study activities were suspended, we modified recruitment strategies to limit in-person contact, shifting toward alternative HIPAA-compliant methods such as clinician referrals, institutional social media, and telemedicine screening and consent procedures. Protocol changes included reducing the frequency of in-person visits, leveraging clinical care visits to collect biospecimens, expanded self-collection of samples at home, and making study materials available online.
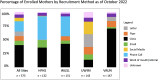
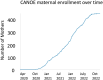
Results: Remote methods, including targeted social media posts, mailed letters, and email, combined with in-clinic recruitment with modifications for social distancing led to successful recruitment at all sites. Rates of consent have been similar across recruitment sites, with the highest rates of enrollment of mother-infant dyads realized by sites that implemented multiple recruitment strategies.
Conclusions: Study procedures that prioritize health and safety measures such as social distancing, study participant convenience, and use diverse recruitment strategies enable successful enrollment of pregnant women and their newborns into clinical research while adhering to public health restrictions during a global pandemic.
Keywords: COVID-19 pandemic; Study recruitment; childhood allergy; pregnancy; protocol redesign; public health; women and infants.
© 2024 Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology.
Conflict of interest statement
This work was supported by the CREW consortium, 10.13039/100000179Office of the Director, 10.13039/100000002National Institutes of Health (NIH; award no. 5UH3 OD023282) and NIH awards UG3 OD035509, UG3 OD035518, UG3 OD035517, UG3 OD035521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Disclosure of potential conflict of interest: T. Hartert reported grants from the National Institutes of Health (NIH) and the 10.13039/100004423World Health Organization during the conduct of the study; and received personal fees from the 10.13039/100001465American Thoracic Society, 10.13039/100004319Pfizer, and Sanofi-Pasteur, outside the submitted work. J. E. Gern reported grants from the NIH during the conduct of the study; and received personal fees from 10.13039/100004325AstraZeneca, outside the submitted work. A. M. Singh reports grants from the NIH during the conduct of the study, and is on the Data Safety Monitoring Board for a Siolta Therapeutics Inc, study. The rest of the authors declare that they have no relevant conflicts of interest.
Figures


References
-
- Ramos S. American Psychological Association; Washington, DC: 2021. COVID-19’s impact felt by researchers: scientists, graduate students talk about conducting research during a pandemic.https://www.apa.org/science/leadership/students/covid-19-impact-researchers (Last accessed May 27, 2024)
-
- Weiner D.L., Balasubramaniam V., Shah S.I., Javier J.R. Pediatric Policy Council. COVID-19 impact on research, lessons learned from COVID-19 research, implications for pediatric research. Pediatr Res. 2020;88:148–150. - PubMed
LinkOut - more resources
Full Text Sources

