Physiological and pathological roles of the thymus and value of thymectomy in myasthenia gravis: a narrative review
- PMID: 38881805
- PMCID: PMC11177005
- DOI: 10.21037/med-23-43
Physiological and pathological roles of the thymus and value of thymectomy in myasthenia gravis: a narrative review
Abstract
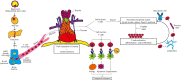
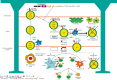
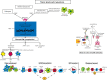
Background and objective: Myasthenia gravis (MG) is a well-elucidated autoimmune disorder affecting the neuromuscular junction. Given the relationship between MG and thymic pathologies, with T cell and antibody-mediated pathogenesis, surgical (i.e., thymectomy) and non-surgical approaches remain a mainstay of management of the disease. This review seeks to outline the involvement of the thymus in the development of lymphocytes leading to MG.
Methods: Different databases were searched exploring the role of thymectomy in treatment and outcomes in various MG patient subpopulations, including in ocular versus generalized disease, different age groups, and antibody status.
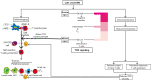
Key content and findings: Overall, the findings of multiple studies and reviews provide evidence to support the efficacy and long-term success of thymectomy in the management of MG; outcomes have included remission status, symptom severity, and need for adjunctive therapy. However, the heterogeneity in the MG population suggests that there are multiple factors that may confound the results of thymectomy and still need further examination. Separately, other autoimmune diseases develop following thymectomy, and further research is required to elucidate this susceptibility. Finally, our review will discuss the different surgical approaches for thymectomy, including their advantages, limitations, and perioperative complications.
Conclusions: Overall, in light of the known pathogenesis and association of the thymus with MG, thymectomy remains an extremely effective approach for long-term management and improved clinical outcomes.
Keywords: Myasthenia gravis (MG); T cell development; thymectomy; thymic pathologies.
2024 Mediastinum. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-43/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Thymectomy and systemic lupus erythematosus (SLE).Ann Ital Chir. 2014 Nov-Dec;85(6):617-8. Ann Ital Chir. 2014. PMID: 25919914
-
Role of thymus on prognosis of myasthenia gravis in Turkish population.North Clin Istanb. 2020 Aug 10;7(5):452-459. doi: 10.14744/nci.2020.51333. eCollection 2020. North Clin Istanb. 2020. PMID: 33163880 Free PMC article.
-
Thymectomy in severe (Myasthenia Gravis Foundation of America classes IV-V) generalized myasthenia gravis: is the game really worth the candle?Eur J Cardiothorac Surg. 2023 May 2;63(5):ezad179. doi: 10.1093/ejcts/ezad179. Eur J Cardiothorac Surg. 2023. PMID: 37162377
-
Effectiveness of thymectomy in juvenile myasthenia gravis and clinical characteristics associated with better outcomes.Neuromuscul Disord. 2021 Nov;31(11):1113-1123. doi: 10.1016/j.nmd.2021.09.013. Epub 2021 Oct 8. Neuromuscul Disord. 2021. PMID: 34756789 Review.
-
An update on thymectomy in myasthenia gravis.Expert Rev Neurother. 2019 Sep;19(9):823-833. doi: 10.1080/14737175.2019.1600404. Epub 2019 Apr 5. Expert Rev Neurother. 2019. PMID: 30917699 Review.
Cited by
-
Multilocular Thymic Cyst with High F18 Fluorodeoxyglucose Uptake and Rheumatoid Arthritis: A Case Report.J Clin Med. 2025 Jan 18;14(2):620. doi: 10.3390/jcm14020620. J Clin Med. 2025. PMID: 39860626 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
