Characterization of anti-drug antibody responses to the T-cell engaging bispecific antibody cibisatamab to understand the impact on exposure
- PMID: 38881900
- PMCID: PMC11176492
- DOI: 10.3389/fimmu.2024.1406353
Characterization of anti-drug antibody responses to the T-cell engaging bispecific antibody cibisatamab to understand the impact on exposure
Abstract
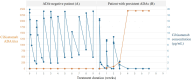
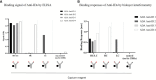
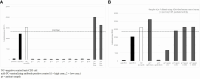
An appropriately designed pharmacokinetic (PK) assay that is sensitive for anti-drug antibody (ADA) impact on relevant exposure is an alternative strategy to understand the neutralizing potential of ADAs. However, guidance on how to develop such PK assays and how to confirm the functional ADA impact on exposure is missing. Here, the PK assay of a T-cell-engaging bispecific antibody, cibisatamab, was developed based on its mechanism of action (MoA). Using critical monoclonal anti-idiotypic (anti-ID) antibody positive controls as ADA surrogates, the impact on exposure was evaluated pre-clinically. In a phase I clinical trial (NCT02324257), initial data suggest that the combination of ADA and PK assays for correlation of the ADA response with cibisatamab exposure. To understand the neutralizing potential of patient-derived ADAs on drug activity, advanced ADA characterization has been performed. Structural binding analysis of ADAs to antibody domains of the drug and its impact on targeting were assessed. For this purpose, relevant patient ADA binding features were identified and compared with the specific monoclonal anti-ID antibody-positive controls. Comparable results of target binding inhibition and similar impacts on exposure suggest that the observed reduction of Cmax and Ctrough levels in patients is caused by the neutralizing potential of ADAs and allows a correlation between ADA response and loss of exposure. Therefore, the described study provides important functional aspects for the development of an appropriately designed PK assay for bispecific antibodies as an alternative option towards understanding the neutralizing ADA impact on exposure.
Keywords: ADA; PK; T cell engager; exposure; immunogenicity.
Copyright © 2024 Lotz, Lutz, Martin-Facklam, Hansbauer, Schick, Moessner, Antony, Stuchly, Viert, Hosse, Freimoser-Grundschober, Klein, Schäfer, Ritter and Stubenrauch.
Conflict of interest statement
CK is employed at F. Hoffman-La Roche AG and holds ownership of stocks and patents of the company. All authors were employees of Roche Diagnostics GmbH or F. Hoffman-La Roche AG during the time this study and associated analyses were being conducted. Authors might hold shares or patents of the company.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

