Digital pathology, deep learning, and cancer: a narrative review
- PMID: 38881914
- PMCID: PMC11170525
- DOI: 10.21037/tcr-23-964
Digital pathology, deep learning, and cancer: a narrative review
Abstract
Background and objective: Cancer is a leading cause of morbidity and mortality worldwide. The emergence of digital pathology and deep learning technologies signifies a transformative era in healthcare. These technologies can enhance cancer detection, streamline operations, and bolster patient care. A substantial gap exists between the development phase of deep learning models in controlled laboratory environments and their translations into clinical practice. This narrative review evaluates the current landscape of deep learning and digital pathology, analyzing the factors influencing model development and implementation into clinical practice.
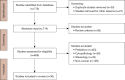
Methods: We searched multiple databases, including Web of Science, Arxiv, MedRxiv, BioRxiv, Embase, PubMed, DBLP, Google Scholar, IEEE Xplore, Semantic Scholar, and Cochrane, targeting articles on whole slide imaging and deep learning published from 2014 and 2023. Out of 776 articles identified based on inclusion criteria, we selected 36 papers for the analysis.
Key content and findings: Most articles in this review focus on the in-laboratory phase of deep learning model development, a critical stage in the deep learning lifecycle. Challenges arise during model development and their integration into clinical practice. Notably, lab performance metrics may not always match real-world clinical outcomes. As technology advances and regulations evolve, we expect more clinical trials to bridge this performance gap and validate deep learning models' effectiveness in clinical care. High clinical accuracy is vital for informed decision-making throughout a patient's cancer care.
Conclusions: Deep learning technology can enhance cancer detection, clinical workflows, and patient care. Challenges may arise during model development. The deep learning lifecycle involves data preprocessing, model development, and clinical implementation. Achieving health equity requires including diverse patient groups and eliminating bias during implementation. While model development is integral, most articles focus on the pre-deployment phase. Future longitudinal studies are crucial for validating models in real-world settings post-deployment. A collaborative approach among computational pathologists, technologists, industry, and healthcare providers is essential for driving adoption in clinical settings.
Keywords: Artificial intelligence (AI); cancer; computational pathology; deep learning (DL); digital pathology (DP).
2024 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-964/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- WHO International Agency for Research on Cancer. Estimated number of deaths in 2020, all cancers, sexes, and ages. Cancer today. 2020. Available online: https://gco.iarc.fr/today/online-analysis-pie
-
- American Cancer Society. The global cancer burden why global cancer rates are rising. Available online: https://rb.gy/8ztpz6
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous