Proteomic analysis and functional validation reveal distinct therapeutic capabilities related to priming of mesenchymal stromal/stem cells with IFN-γ and hypoxia: potential implications for their clinical use
- PMID: 38882056
- PMCID: PMC11179434
- DOI: 10.3389/fcell.2024.1385712
Proteomic analysis and functional validation reveal distinct therapeutic capabilities related to priming of mesenchymal stromal/stem cells with IFN-γ and hypoxia: potential implications for their clinical use
Abstract
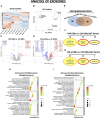
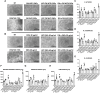
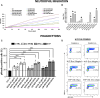
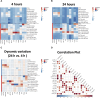
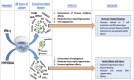
Mesenchymal stromal/stem cells (MSCs) are a heterogeneous population of multipotent cells that can be obtained from various tissues, such as dental pulp, adipose tissue, bone marrow and placenta. MSCs have gained importance in the field of regenerative medicine because of their promising role in cell therapy and their regulatory abilities in tissue repair and regeneration. However, a better characterization of these cells and their products is necessary to further potentiate their clinical application. In this study, we used unbiased high-resolution mass spectrometry-based proteomic analysis to investigate the impact of distinct priming strategies, such as hypoxia and IFN-γ treatment, on the composition and therapeutic functionality of the secretome produced by MSCs derived from the amniotic membrane of the human placenta (hAMSCs). Our investigation revealed that both types of priming improved the therapeutic efficacy of hAMSCs, and these improvements were related to the secretion of functional factors present in the conditioned medium (CM) and exosomes (EXOs), which play crucial roles in mediating the paracrine effects of MSCs. In particular, hypoxia was able to induce a pro-angiogenic, innate immune response-activating, and tissue-regenerative hAMSC phenotype, as highlighted by the elevated production of regulatory factors such as VEGFA, PDGFRB, ANGPTL4, ENG, GRO-γ, IL8, and GRO-α. IFN-γ priming, instead, led to an immunosuppressive profile in hAMSCs, as indicated by increased levels of TGFB1, ANXA1, THBS1, HOMER2, GRN, TOLLIP and MCP-1. Functional assays validated the increased angiogenic properties of hypoxic hAMSCs and the enhanced immunosuppressive activity of IFN-γ-treated hAMSCs. This study extends beyond the direct priming effects on hAMSCs, demonstrating that hypoxia and IFN-γ can influence the functional characteristics of hAMSC-derived secretomes, which, in turn, orchestrate the production of functional factors by peripheral blood cells. This research provides valuable insights into the optimization of MSC-based therapies by systematically assessing and comparing the priming type-specific functional features of hAMSCs. These findings highlight new strategies for enhancing the therapeutic efficacy of MSCs, particularly in the context of multifactorial diseases, paving the way for the use of hAMSC-derived products in clinical practice.
Keywords: IFN-γ priming; MSC paracrine effects; MSC priming; MSC therapeutic properties; hypoxia priming; placenta-derived mesenchymal stromal/stem cells; proteomic analysis.
Copyright © 2024 Calligaris, Zito, Busà, Bulati, Iannolo, Gallo, Carreca, Cuscino, Castelbuono, Carcione, Centi, Amico, Bertani, Chinnici, Conaldi, Scilabra and Miceli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bulati M., Gallo A., Zito G., Busa R., Iannolo G., Cuscino N., et al. (2023). 3D culture and interferon-gamma priming modulates characteristics of mesenchymal stromal/stem cells by modifying the expression of both intracellular and exosomal microRNAs. Biol. (Basel) 12 (8), 1063. 10.3390/biology12081063 - DOI - PMC - PubMed
-
- Bulati M., Miceli V., Gallo A., Amico G., Carcione C., Pampalone M., et al. (2020). The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-gamma produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front. Immunol. 11, 54. 10.3389/fimmu.2020.00054 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

