MoS2, WS2, and MoWS2 Flakes as Reversible Host Materials for Sodium-Ion and Potassium-Ion Batteries
- PMID: 38882118
- PMCID: PMC11170725
- DOI: 10.1021/acsomega.4c01966
MoS2, WS2, and MoWS2 Flakes as Reversible Host Materials for Sodium-Ion and Potassium-Ion Batteries
Abstract
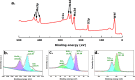
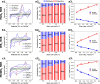
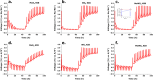
Transition-metal dichalcogenides (TMDs) and their alloys are vital for the development of sustainable and economical energy storage alternatives due to their large interlayer spacing and hosting ability for alkali-metal ions. Although the Li-ion chemically correlates with the Na-ion and K-ion, research on batteries with TMD anodes for K+ is still in its infancy. This research explores TMDs such as molybdenum disulfide (MoS2) and tungsten disulfide (WS2) and TMD alloys such as molybdenum tungsten disulfide (MoWS2) for both sodium-ion batteries (NIBs) and potassium-ion batteries (KIBs). The cyclic stability test analysis indicates that in the initial cycle, the MoS2 NIB demonstrates exceptional performance, with a peak charge capacity of 1056 mAh g-1, while retaining high Coulombic efficiency. However, the WS2 KIB underperforms, with the least charge capacity of 130 mAh g-1 in the first cycle and exceptionally low retention at a current density of 100 mA g-1. The MoWS2 TMD alloy exhibits a moderate charge capacity and cyclic efficiency for both NIBs and KIBs. This comparison study shows that decreasing sizes of alkali-metal ions and constituent elements in TMDs or TMD alloys leads to decreased resistance and slower degradation processes as indicated by cyclic voltammetry and electrochemical impedance spectroscopy after 10 cycles. Furthermore, the study of probable electrochemical intercalation and removal processes of Na-ions and K-ions demonstrates that large geometrically shaped TMD flakes are more responsive to intercalation for Na-ions than K-ions. These performance comparisons of different TMD materials for NIBs and KIBs may promote the future development of these batteries.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
WS2 Nanosheet Loaded Silicon-Oxycarbide Electrode for Sodium and Potassium Batteries.Nanomaterials (Basel). 2022 Nov 25;12(23):4185. doi: 10.3390/nano12234185. Nanomaterials (Basel). 2022. PMID: 36500808 Free PMC article.
-
Superior electrochemical performance of layered WTe2 as potassium-ion battery electrode.Nanotechnology. 2020 Nov 6;31(45):455406. doi: 10.1088/1361-6528/ababcc. Epub 2020 Aug 3. Nanotechnology. 2020. PMID: 32746438
-
Superior Potassium Ion Storage via Vertical MoS2 "Nano-Rose" with Expanded Interlayers on Graphene.Small. 2017 Nov;13(42). doi: 10.1002/smll.201701471. Epub 2017 Sep 22. Small. 2017. PMID: 28941005
-
Recent Advances in Layered Metal-Oxide Cathodes for Application in Potassium-Ion Batteries.Adv Sci (Weinh). 2022 Jun;9(18):e2105882. doi: 10.1002/advs.202105882. Epub 2022 Apr 27. Adv Sci (Weinh). 2022. PMID: 35478355 Free PMC article. Review.
-
How does Molybdenum Disulfide Store Charge: A Minireview.ChemSusChem. 2020 Mar 20;13(6):1354-1365. doi: 10.1002/cssc.201903320. Epub 2020 Feb 4. ChemSusChem. 2020. PMID: 32017468 Review.
References
-
- Zhang W.; Lu J.; Guo Z. Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today 2021, 50, 400–417. 10.1016/j.mattod.2021.03.015. - DOI
LinkOut - more resources
Full Text Sources
