A Comparative DFT Investigation of the Adsorption of Temozolomide Anticancer Drug over Beryllium Oxide and Boron Nitride Nanocarriers
- PMID: 38882172
- PMCID: PMC11170632
- DOI: 10.1021/acsomega.4c02882
A Comparative DFT Investigation of the Adsorption of Temozolomide Anticancer Drug over Beryllium Oxide and Boron Nitride Nanocarriers
Abstract
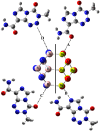
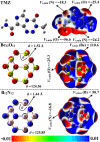
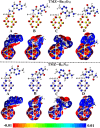
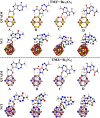
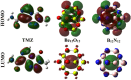
Herein, attempts were made to explore the adsorption prospective of beryllium oxide (Be12O12) and boron nitride (B12N12) nanocarriers toward the temozolomide (TMZ) anticancer drug. A systematic investigation of the TMZ adsorption over nanocarriers was performed by using quantum chemical density functional theory (DFT). The favorability of Be12O12 and B12N12 nanocarriers toward loading TMZ was investigated through A↔D configurations. Substantial energetic features of the proposed configurations were confirmed by negative adsorption (E ads) energy values of up to -30.47 and -26.94 kcal/mol for TMZ•••Be12O12 and •••B12N12 complexes within configuration A, respectively. As per SAPT results, the dominant contribution beyond the studied adsorptions was found for the electrostatic forces (E elst = -100.21 and -63.60 kcal/mol for TMZ•••B12N12 and •••Be12O12 complexes within configuration A, respectively). As a result of TMZ adsorption, changes in the energy of molecular orbitals followed by alterations in global reactivity descriptors were observed. Various intermolecular interactions within the studied complexes were assessed by QTAIM analysis. Notably, a favorable adsorption process was also observed under the effect of water with adsorption energy ( reaching -28.05 and -22.26 kcal/mol for TMZ•••B12N12 and •••Be12O12 complexes within configuration A, respectively. The drug adsorption efficiency of the studied nanocarriers was further examined by analyzing the IR and Raman spectra. From a sustained drug delivery point of view, the release pattern of TMZ from the nanocarrier surface was investigated by recovery time calculations. Additionally, the significant role of doping by heavy atoms (i.e., MgBe11O12 and AlB11N12) on the favorability of TMZ adsorption was investigated and compared to pure analogs (i.e., Be12O12 and B12N12). The obtained data from thermodynamic calculations highlighted that the adsorption process over pure and doped nanocarriers was spontaneous and exothermic. The emerging findings provide a theoretical base for future works related to nanocarrier applications in the drug delivery process, especially for the TMZ anticancer drug.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
A DFT investigation on the potential of beryllium oxide (Be12O12) as a nanocarrier for nucleobases.PLoS One. 2024 Nov 22;19(11):e0313885. doi: 10.1371/journal.pone.0313885. eCollection 2024. PLoS One. 2024. PMID: 39576836 Free PMC article.
-
Adsorption of Chlormethine Anti-Cancer Drug on Pure and Aluminum-Doped Boron Nitride Nanocarriers: A Comparative DFT Study.Pharmaceuticals (Basel). 2022 Sep 23;15(10):1181. doi: 10.3390/ph15101181. Pharmaceuticals (Basel). 2022. PMID: 36297293 Free PMC article.
-
Adsorption of Molnupiravir anti-COVID-19 drug over B12N12 and Al12N12 nanocarriers: a DFT study.J Biomol Struct Dyn. 2023;41(22):12923-12937. doi: 10.1080/07391102.2023.2169763. Epub 2023 Jan 23. J Biomol Struct Dyn. 2023. PMID: 36688358
-
A density functional theory study on the adsorption of Mercaptopurine anti-cancer drug and Cu/Zn-doped boron nitride nanocages as a drug delivery.J Biomol Struct Dyn. 2024 Feb-Mar;42(4):1647-1654. doi: 10.1080/07391102.2023.2212788. Epub 2023 May 18. J Biomol Struct Dyn. 2024. PMID: 37199275 Review.
-
Improving temozolomide biopharmaceutical properties in glioblastoma multiforme (GBM) treatment using GBM-targeting nanocarriers.Eur J Pharm Biopharm. 2021 Nov;168:76-89. doi: 10.1016/j.ejpb.2021.08.011. Epub 2021 Aug 28. Eur J Pharm Biopharm. 2021. PMID: 34461214 Review.
Cited by
-
A DFT investigation on the potential of beryllium oxide (Be12O12) as a nanocarrier for nucleobases.PLoS One. 2024 Nov 22;19(11):e0313885. doi: 10.1371/journal.pone.0313885. eCollection 2024. PLoS One. 2024. PMID: 39576836 Free PMC article.
-
Repurposing fluoroquinolones as cancer chemosensitizers: a way to overcome cancer therapeutic bottleneck.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug 11. doi: 10.1007/s00210-025-04508-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40788483 Review.
References
-
- Chatterjee P.; Kumar S. Current developments in nanotechnology for cancer treatment. Mater. Today: proc. 2022, 48, 1754–1758. 10.1016/j.matpr.2021.10.048. - DOI
-
- Petranovska A. L.; Abramov N. V.; Turanska S. P.; Gorbyk P. P.; Kaminskiy A. N.; Kusyak N. V. Adsorption of cis-dichlorodiammineplatinum by nanostructures based on single-domain magnetite. J. Nanostructure Chem. 2015, 5, 275–285. 10.1007/s40097-015-0159-9. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
